|
Chromate Conversion Coating
Chromate conversion coating or alodine coating is a type of conversion coating used to passivate steel, aluminium, zinc, cadmium, copper, silver, titanium, magnesium, and tin alloys. The coating serves as a corrosion inhibitor, as a primer to improve the adherence of paints and adhesives, as a decorative finish, or to preserve electrical conductivity. It also provides some resistance to abrasion and light chemical attack (such as dirty fingers) on soft metals. Chromate conversion coatings are commonly applied to items such as screws, hardware and tools. They usually impart a distinctively iridescent, greenish-yellow color to otherwise white or gray metals. The coating has a complex composition including chromium salts, and a complex structure. The process is sometimes called alodine coating, a term used specifically in reference to the trademarked Alodine process of Henkel Surface Technologies. Process Chromate conversion coatings are usually applied by immersing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Screw
A screw is an externally helical threaded fastener capable of being tightened or released by a twisting force (torque) to the screw head, head. The most common uses of screws are to hold objects together and there are many forms for a variety of materials. Screws might be inserted into holes in assembled parts or a screw may form its own thread. The #Differentiation between bolt and screw, difference between a screw and a bolt is that the latter is designed to be tightened or released by torquing a Nut (hardware), nut. The screw head on one end has a slot or other feature that commonly requires a tool to transfer the twisting force. Common tools for driving screws include screwdrivers, wrenches, coins and hex keys. The head is usually larger than the body, which provides a ''bearing surface'' and keeps the screw from being driven deeper than its length; an exception being the ''set screw'' (aka grub screw). The cylindrical portion of the screw from the underside of the head t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redox Reaction
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction. There are two classes of redox reactions: * Electron-transfer – Only one (usually) electron flows from the atom, ion, or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * Atom transfer – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously, the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuric Acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, and hydrogen, with the molecular formula . It is a colorless, odorless, and Viscosity, viscous liquid that is Miscibility, miscible with water. Pure sulfuric acid does not occur naturally due to its Dehydration reaction, strong affinity to water vapor; it is Hygroscopy, hygroscopic and readily absorbs water vapor from the Atmosphere of Earth, air. Concentrated sulfuric acid is a strong oxidant with powerful dehydrating properties, making it highly corrosive towards other materials, from rocks to metals. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid but, to the contrary, dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is releas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Milliliter
The litre (Commonwealth English, Commonwealth spelling) or liter (American English, American spelling) (SI symbols L and l, other symbol used: ℓ) is a metric units, metric unit of volume. It is equal to 1 cubic decimetre (dm3), 1000 cubic centimetres (cm3) or 0.001 cubic metres (m3). A cubic decimetre (or litre) occupies a volume of (see figure) and is thus equal to one-thousandth of a cubic metre. The original French metric system used the litre as a SI base unit, base unit. The word ''litre'' is derived from an older French unit, the ''Units of measurement in France before the French Revolution#Volume – Dry measures, litron'', whose name came from Byzantine Greek language, Greek—where it was a unit of weight, not volume—via Late Medieval Latin, and which equalled approximately 0.831 litres. The litre was also used in several subsequent versions of the metric system and is accepted for use with the SI, despite it not being an International System of Units, SI un ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Dichromate
Sodium dichromate is the inorganic compound with the formula Na2 Cr2 O7. However, the salt is usually handled as its dihydrate Na2Cr2O7·2 H2O. Virtually all chromium ore is processed via conversion to sodium dichromate and virtually all compounds and materials based on chromium are prepared from this salt.Gerd Anger, Jost Halstenberg, Klaus Hochgeschwender, Christoph Scherhag, Ulrich Korallus, Herbert Knopf, Peter Schmidt, Manfred Ohlinger, "Chromium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. In terms of reactivity and appearance, sodium dichromate and potassium dichromate are very similar. The sodium salt is, however, around twenty times more soluble in water than the potassium salt (49 g/L at 0 °C) and its equivalent weight is also lower, which is often desirable.Freeman, F. "Sodium Dichromate" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. . Preparation Sodium dichromate i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liter
The litre ( Commonwealth spelling) or liter ( American spelling) (SI symbols L and l, other symbol used: ℓ) is a metric unit of volume. It is equal to 1 cubic decimetre (dm3), 1000 cubic centimetres (cm3) or 0.001 cubic metres (m3). A cubic decimetre (or litre) occupies a volume of (see figure) and is thus equal to one-thousandth of a cubic metre. The original French metric system used the litre as a base unit. The word ''litre'' is derived from an older French unit, the '' litron'', whose name came from Byzantine Greek—where it was a unit of weight, not volume—via Late Medieval Latin, and which equalled approximately 0.831 litres. The litre was also used in several subsequent versions of the metric system and is accepted for use with the SI, despite it not being an SI unit. The SI unit of volume is the cubic metre (m3). The spelling used by the International Bureau of Weights and Measures is "litre", [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gram
The gram (originally gramme; SI unit symbol g) is a Physical unit, unit of mass in the International System of Units (SI) equal to one thousandth of a kilogram. Originally defined in 1795 as "the absolute Mass versus weight, weight of a volume of pure water equal to Cube (algebra), the cube of the hundredth part of a metre [1 Cubic centimetre, cm3], and at Melting point of water, the temperature of Melting point, melting ice", the defining temperature (0 °C) was later changed to the temperature of maximum density of water (approximately 4 °C). Subsequent redefinitions agree with this original definition to within 30 Parts-per notation, parts per million (0.003%), with the maximum density of water remaining very close to 1 g/cm3, as shown by modern measurements. By the late 19th century, there was an effort to make the Base unit (measurement), base unit the kilogram and the gram a derived unit. In 1960, the new International System of Units defined a '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cronak Process
The Cronak process is a conventional chromate conversion coating process developed in 1933 by The New Jersey Zinc Company. It involves immersing a zinc or zinc-plated article for 5 to 15 seconds in a chromate solution, typically prepared from sodium dichromate and sulfuric acid. The process was patented in the United States on March 24, 1936 with USPTO The United States Patent and Trademark Office (USPTO) is an agency in the U.S. Department of Commerce that serves as the national patent office and trademark registration authority for the United States. The USPTO's headquarters are in Ale ... number 2,035,380. References {{Reflist External links * https://web.archive.org/web/20141211093127/http://www.innovateus.net/science/what-zinc-chromate-used Chemical processes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
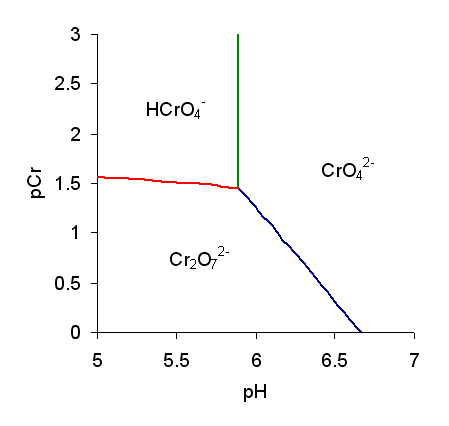
Chromate And Dichromate
Chromate salts contain the chromate anion, . Dichromate salts contain the dichromate anion, . They are oxyanions of chromium in the +6 oxidation state and are moderately strong oxidizing agents. In an aqueous solution, chromate and dichromate ions can be interconvertible. Chemical properties Potassium-chromate-sample.jpg, Potassium chromate Potassium-dichromate-sample.jpg, Potassium dichromate Chromates react with hydrogen peroxide, giving products in which peroxide, , replaces one or more oxygen atoms. In acid solution the unstable blue peroxo complex Chromium(VI) oxide peroxide, , is formed; it is an uncharged covalent molecule, which may be extracted into ether. Addition of pyridine results in the formation of the more stable complex . Acid–base properties In aqueous solution, chromate and dichromate anions exist in a chemical equilibrium. : The predominance diagram shows that the position of the equilibrium depends on both pH and the analytical concentration o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexavalent Chromium
Hexavalent chromium (chromium(VI), Cr(VI), chromium 6) is any chemical compound that contains the element chromium in the +6 oxidation state (thus hexavalent). It has been identified as carcinogenic, which is of concern since approximately of hexavalent chromium were produced in 1985. Hexavalent chromium compounds can be carcinogens ( IARC Group 1), especially if airborne and inhaled where they can cause lung cancer. Occurrence and uses Hexavalent chromium occurs only rarely in nature, an exception being crocoite (PbCrO4). It is however produced on a large scale industrially. Virtually all chromium ore is processed via the formation of hexavalent chromium, specifically the salt sodium dichromate. Sodium chromate is converted into other hexavalent chromium compounds such as chromium trioxide and various salts of chromate and dichromate. Industrial uses of hexavalent chromium compounds include chromate pigments in dyes, paints, inks, and plastics; chromates added as anti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrophobe
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water. Hydrophobic molecules tend to be nonpolar and, thus, prefer other neutral molecules and nonpolar solvents. Because water molecules are polar, hydrophobes do not dissolve well among them. Hydrophobic molecules in water often cluster together, forming micelles. Water on hydrophobic surfaces will exhibit a high contact angle. Examples of hydrophobic molecules include the alkanes, oils, fats, and greasy substances in general. Hydrophobic materials are used for oil removal from water, the management of oil spills, and chemical separation processes to remove non-polar substances from polar compounds. The term ''hydrophobic''—which comes from the Ancient Greek (), "having a fear of water", constructed Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon. revised and augmented throu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |