|
Brønsted Catalysis Equation
The Brønsted catalysis equation or law of correlation, after Johannes Nicolaus Brønsted, gives the relationship between acid strength and catalytic activity in general acid catalysis.Brønsted, J. N.; Pedersen, K. J. Zeitschrift für Phys. Chemie, Stöchiometrie und Verwandtschaftslehre 1924, 108, 185–235. \ \log k = \alpha*\log(K_a) + C A plot of the common logarithm of the reaction rate constant k versus the logarithm of the ionization constant Ka for a series of acids (for example a group of substituted phenols or carboxylic acids) gives a straight line with slope α and intercept C. The Brønsted equation is a free-energy relationship. The relationship implies that the Gibbs free energy for proton dissociation is proportional to the activation energy for the catalytic step. When the relationship is not linear, the chosen group of catalysts do not operate through the same reaction mechanism. Specific and general catalysis is also found in base catalysed reactions and b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Johannes Nicolaus Brønsted
Johannes Nicolaus Brønsted (; 22 February 1879 – 17 December 1947) was a Danish physical chemist, who developed the Brønsted–Lowry acid–base theory simultaneously with and independently of Martin Lowry. Biography Brønsted was born in Varde, Denmark on 22 February 1879. His mother died shortly after his birth and at the age of 14, Brønsted lost his father and moved to Copenhagen with his older sister and his stepmother. In 1897, Brønsted began his studies as a chemical engineer at the Polytechnic Institute in Copenhagen. After his first degree, Brønsted changed fields and received his magister degree in chemistry in 1902 from the University of Copenhagen. In 1905, he became an assistant at the Chemical Institute and obtained his doctoral degree in 1908. In the same year, Brønsted became a professor of physical and inorganic chemistry at the University of Copenhagen. In 1929, Brønsted was a visiting professor at Yale University. His research gained worldwide reco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenalene
1''H''-Phenalene, often called simply phenalene is a polycyclic aromatic hydrocarbon (PAH). Like many PAHs, it is an atmospheric pollutant formed during the combustion of fossil fuels. It is the parent compound for the phosphorus-containing phosphaphenalenes. Reactions Phenalene is deprotonated by potassium methoxide to give the phenalenyl anion. See also * Zethrene * Cyclopentadiene Cyclopentadiene is an organic compound with the formula C5H6.LeRoy H. Scharpen and Victor W. Laurie (1965): "Structure of cyclopentadiene". ''The Journal of Chemical Physics'', volume 43, issue 8, pages 2765-2766. It is often abbreviated CpH beca ... References Polycyclic aromatic hydrocarbons Tricyclic compounds {{hydrocarbon-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Open Access (publishing)
Open access (OA) is a set of principles and a range of practices through which research outputs are distributed online, free of access charges or other barriers. With open access strictly defined (according to the 2001 definition), or libre open access, barriers to copying or reuse are also reduced or removed by applying an open license for copyright. The main focus of the open access movement is "peer reviewed research literature". Historically, this has centered mainly on print-based academic journals. Whereas non-open access journals cover publishing costs through access tolls such as subscriptions, site licenses or pay-per-view charges, open-access journals are characterised by funding models which do not require the reader to pay to read the journal's contents, relying instead on author fees or on public funding, subsidies and sponsorships. Open access can be applied to all forms of published research output, including peer-reviewed and non peer-reviewed academic journal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arkivoc
''Arkivoc'' (''Archive for Organic Chemistry'') is a peer-reviewed open access scientific journal covering all aspects of organic chemistry. It is published by the non-profit organization Arkat USA, which was established in 2000 through a personal donation from Alan R. Katritzky and Linde Katritzky. ''Arkivoc'' is the primary publication of Arkat USA. According to the ''Journal Citation Reports'', the journal has a 2014 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as ... of 1.165, ranking it 37th out of 57 journals in the category "Chemistry, Organic". Abstracting and Indexing According to the Journal Citation Reports, the journal has a 2018 impact factor of 1.253. The journal is indexed in Web of Science: Science Citation Index Expanded. References External lin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Andrew Streitwieser
Andrew Streitwieser was an American chemist known for his contributions to physical organic chemistry. Streitwieser was born in 1927 in Buffalo, New York and he grew up in New York City. He attended Columbia College and then Columbia University where he earned a PhD in the research group of William von Eggers Doering in 1952.Michigan State University. Department of Chemistry. Portraits. Andrew Streitwieser, Jr. retrieved Aug. 11, 2018. He then was a postdoctoral fellow in the laboratory of |
Notes
Note, notes, or NOTE may refer to: Music and entertainment * Musical note, a pitched sound (or a symbol for a sound) in music * ''Notes'' (album), a 1987 album by Paul Bley and Paul Motian * ''Notes'', a common (yet unofficial) shortened version of the title of the American TV situation comedy, '' Notes from the Underbelly'' * ''Notes'' (film), a short by John McPhail * ''Notes'' (journal), the quarterly journal of the Music Library Association Finance * Banknote, a form of cash currency, also known as ''bill'' in the United States and Canada * Promissory note, a contract binding one party to pay money to a second party * Note, a security (finance), a type of bond Technology and science * IBM Notes, (formerly Lotus Notes), a client-server, collaborative application owned by IBM Software Group * Natural orifice transluminal endoscopic surgery (NOTES), a type of minimally invasive surgery * Notes (Apple), a note-taking application bundled with macOS and iOS * Notes, another ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bell–Evans–Polanyi Principle
In physical chemistry, the Evans–Polanyi principle (also referred to as the Bell–Evans–Polanyi principle, Brønsted–Evans–Polanyi principle, or Evans–Polanyi–Semenov principle) observes that the difference in activation energy between two reactions of the same family is proportional to the difference of their enthalpy of reaction. This relationship can be expressed as : E_\text = E_0 + \alpha \Delta H, where : E_\text is the activation energy of a reference reaction of the same class, : \Delta H is the enthalpy of reaction, : \alpha characterizes the position of the transition state along the reaction coordinate (such that 0 \leq \alpha \leq 1). The Evans–Polanyi model is a linear energy relationship that serves as an efficient way to calculate activation energy of many reactions within a distinct family. The activation energy may be used to characterize the kinetic rate parameter of a given reaction through application of the Arrhenius equation. The Evans� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Free-energy Relationship
In physical organic chemistry, a free-energy relationship or Gibbs energy relation relates the logarithm of a reaction rate constant or equilibrium constant for one series of chemical reactions with the logarithm of the rate or equilibrium constant for a related series of reactions. Free energy relationships establish the extent at which bond formation and breakage happen in the transition state of a reaction, and in combination with kinetic isotope experiments a reaction mechanism can be determined. Free energy relationships are often used to calculate equilibrium constants since they are experimentally difficult to determine. The most common form of free-energy relationships are linear free-energy relationships (LFER). The Brønsted catalysis equation describes the relationship between the ionization constant of a series of catalysts and the reaction rate constant for a reaction on which the catalyst operates. The Hammett equation predicts the equilibrium constant or reaction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
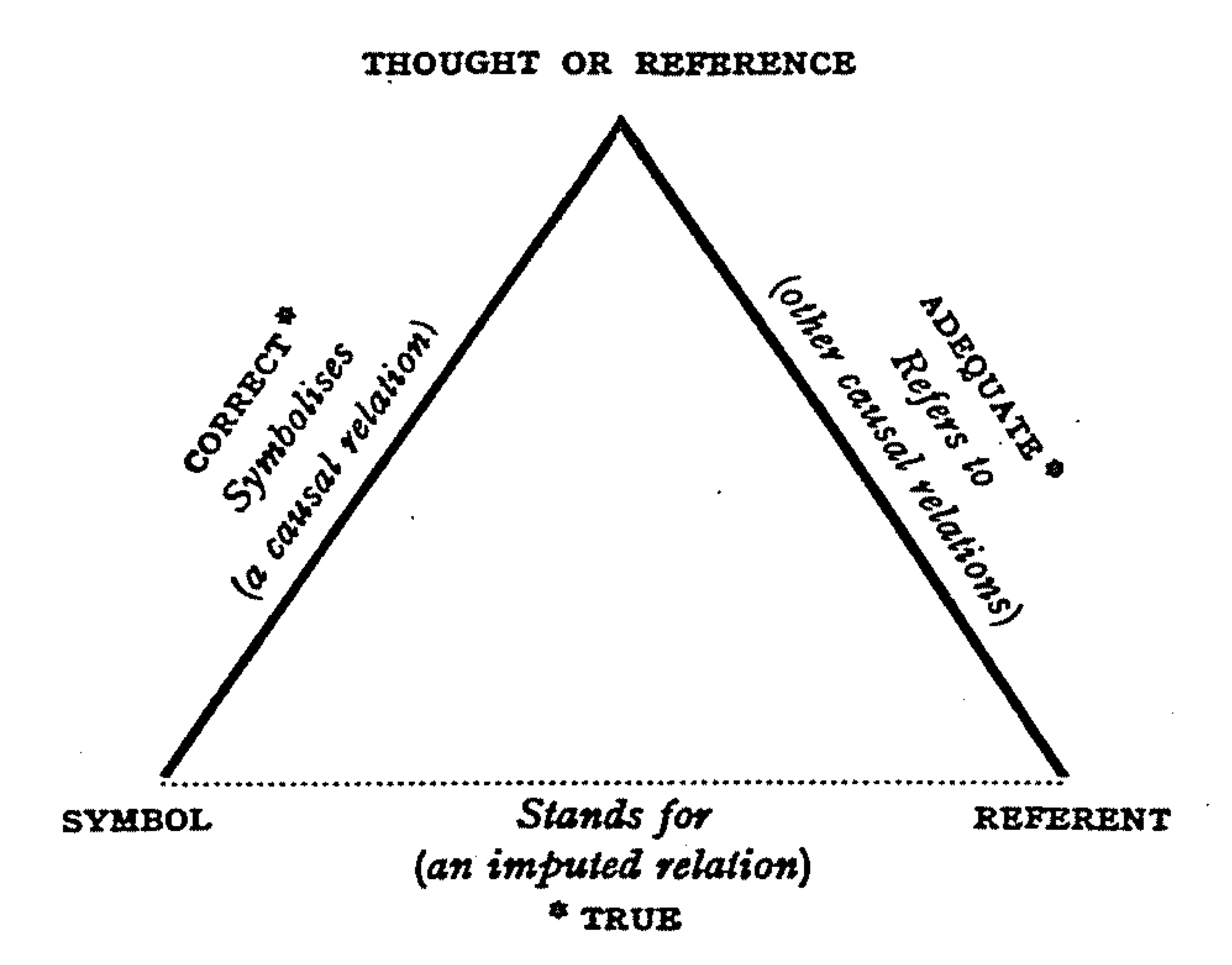
References
Reference is a relationship between objects in which one object designates, or acts as a means by which to connect to or link to, another object. The first object in this relation is said to ''refer to'' the second object. It is called a ''name'' for the second object. The second object, the one to which the first object refers, is called the ''referent'' of the first object. A name is usually a phrase or expression, or some other symbolic representation. Its referent may be anything – a material object, a person, an event, an activity, or an abstract concept. References can take on many forms, including: a thought, a sensory perception that is audible (onomatopoeia), visual (text), olfactory, or tactile, emotional state, relationship with other, spacetime coordinate, symbolic or alpha-numeric, a physical object or an energy projection. In some cases, methods are used that intentionally hide the reference from some observers, as in cryptography. References feature in many s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylene
In organic chemistry, the phenylene group () is based on a di-substituted benzene ring ( arylene). For example, poly(''p''-phenylene) is a polymer A polymer (; Greek '' poly-'', "many" + ''-mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ... built up from ''para''-phenylene repeating units.p. C-9, Section 11.6, Handbook of Chemistry and Physics, 62nd Edition, 1981-1982, CRC Press The phenylene group has three structural isomers, based on which hydrogens are substituted: ''para''-phenylene, ''meta''-phenylene, and ''ortho''-phenylene. References Arenediyl groups {{Aromatic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indene
Indene is a flammable polycyclic hydrocarbon with chemical formula . It is composed of a benzene ring fused with a cyclopentene ring. This aromatic liquid is colorless although samples often are pale yellow. The principal industrial use of indene is in the production of indene/coumarone thermoplastic resins. Substituted indenes and their closely related indane derivatives are important structural motifs found in many natural products and biologically active molecules, such as sulindac. Isolation Indene occurs naturally in coal-tar fractions boiling around 175–185 °C. It can be obtained by heating this fraction with sodium to precipitate solid "sodio-indene". This step exploits indene's weak acidity evidenced by its deprotonation by sodium to give the indenyl derivative. The sodio-indene is converted back to indene by steam distillation. Reactivity Indene readily polymerises. Oxidation of indene with acid dichromate yields homophthalic acid (''o''-carboxylphenyla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition State
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked with the double dagger ‡ symbol. As an example, the transition state shown below occurs during the SN2 reaction of bromoethane with a hydroxide anion: The activated complex of a reaction can refer to either the transition state or to other states along the reaction coordinate between reactants and products, especially those close to the transition state. Peter Atkins and Julio de Paula, ''Physical Chemistry'' (8th ed., W.H. Freeman 2006), p.809 According to the transition state theory, once the reactants have passed through the transition state configuration, they always continue to form products. History of concept The concept of a transition state has been important in many theories of the rates at which chemical reactio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |