|
Bismuth Compounds
Bismuth compounds are compounds containing the element bismuth (Bi). Bismuth forms trivalent and pentavalent compounds, the trivalent ones being more common. Many of its chemical properties are similar to those of arsenic and antimony, although they are less toxic than derivatives of those lighter elements. Oxides and sulfides At elevated temperatures, the vapors of the metal combine rapidly with oxygen, forming the yellow trioxide, . Wiberg, p. 768. Greenwood, p. 553. When molten, at temperatures above 710 °C, this oxide corrodes any metal oxide and even platinum. Krüger, p. 185 On reaction with a base, it forms two series of oxyanions: , which is polymeric and forms linear chains, and . The anion in is a cubic octameric anion, , whereas the anion in is tetrameric. The dark red bismuth(V) oxide, , is unstable, liberating gas upon heating. The compound NaBiO3 is a strong oxidising agent. Greenwood, p. 578. Bismuth sulfide, , occurs naturally in bismuth ores. It i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bismuth(III) Oxide 2
Bismuth is a chemical element with the Symbol (chemistry), symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs naturally, and its sulfide and oxide forms are important commercial ores. The free element is 86% as dense as lead. It is a brittle metal with a silvery-white color when freshly produced. Passivation (chemistry), Surface oxidation generally gives samples of the metal a somewhat rosy cast. Further oxidation under heat can give bismuth a vividly Iridescence, iridescent appearance due to thin-film interference. Bismuth is both the most Diamagnetism, diamagnetic element and one of the least Thermal conductivity, thermally conductive metals known. Bismuth was long considered the element with the highest atomic mass whose nuclei do not spontaneously decay. However, in 2003 it was discovered to be extremely weakly radioactive. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bismuthine
Bismuthine (IUPAC name: bismuthane) is the chemical compound with the formula BiH3. As the heaviest analogue of ammonia (a pnictogen hydride), BiH3 is unstable, decomposing to bismuth metal well below 0 °C. This compound adopts the expected pyramidal structure with H–Bi–H angles of around 90°. The term ''bismuthine'' may also refer to a member of the family of organobismuth(III) species having the general formula , where R is an organic substituent. For example, Bi(CH3)3 is ''trimethylbismuthine''. Preparation and properties BiH3 is prepared by the redistribution of methylbismuthine (BiH2Me):Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001.. :3 BiH2Me → 2 BiH3 + BiMe3 The required BiH2Me, which is also thermally unstable, is generated by reduction of methylbismuth dichloride, BiCl2Me with LiAlH4. As suggested by the behavior of SbH3, BiH3 is unstable and decomposes to its constituent elements according to the following equatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hafnium(IV) Chloride
Hafnium(IV) chloride is the inorganic compound with the formula HfCl4. This colourless solid is the precursor to most hafnium organometallic compounds. It has a variety of highly specialized applications, mainly in materials science and as a catalyst. Preparation HfCl4 can be produced by several related procedures: *The reaction of carbon tetrachloride and hafnium oxide at above 450 °C; :HfO2 + 2 CCl4 → HfCl4 + 2 COCl2 *Chlorination of a mixture of HfO2 and carbon above 600 °C using chlorine gas or sulfur monochloride: :HfO2 + 2 Cl2 + C → HfCl4 + CO2 *Chlorination of hafnium carbide above 250 °C. Separation of Zr and Hf Hafnium and zirconium occur together in minerals such as zircon, cyrtolite and baddeleyite. Zircon contains 0.05% to 2.0% hafnium dioxide HfO2, cyrtolite with 5.5% to 17% HfO2 and baddeleyite contains 1.0 to 1.8 percent HfO2.Newnham, Ivan Edgar "Purification of Hafnium Tetrachloride". November 22, 1960. Hafnium and zircon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trigonal Prism
In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The octahedron has eight faces, hence the Prefix (linguistics), prefix ''wiktionary:octa-, octa''. The octahedron is one of the Platonic solids, although octahedral molecules typically have an atom in their centre and no bonds between the ligand atoms. A perfect octahedron belongs to the point group Oh. Examples of octahedral compounds are sulfur hexafluoride SF6 and molybdenum hexacarbonyl Mo(CO)6. The term "octahedral" is used somewhat loosely by chemists, focusing on the geometry of the bonds to the central atom and not considering differences among the ligands themselves. For example, Cobalt(III) hexammine chloride, , which is not octahedral in the mathematical sense due to the orientation of the bonds, is referred to as octahedral. The con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluoride, chloride, bromide, iodide, astatide, or theoretically tennesside compound. The alkali metals combine directly with halogens under appropriate conditions forming halides of the general formula, MX (X = F, Cl, Br or I). Many salts are halides; the ''hal-'' syllable in ''halide'' and ''halite'' reflects this correlation. All Group 1 metals form halides that are white solids at room temperature. A halide ion is a halogen atom bearing a negative charge. The halide anions are fluoride (), chloride (), bromide (), iodide () and astatide (). Such ions are present in all ionic halide salts. Halide minerals contain halides. All these halides are colourless, high melting crystalline solids having high negative enthalpies of format ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spintronics
Spintronics (a portmanteau meaning spin transport electronics), also known as spin electronics, is the study of the intrinsic spin of the electron and its associated magnetic moment, in addition to its fundamental electronic charge, in solid-state devices. The field of spintronics concerns spin-charge coupling in metallic systems; the analogous effects in insulators fall into the field of multiferroics. Spintronics fundamentally differs from traditional electronics in that, in addition to charge state, electron spins are exploited as a further degree of freedom, with implications in the efficiency of data storage and transfer. Spintronic systems are most often realised in dilute magnetic semiconductors (DMS) and Heusler alloys and are of particular interest in the field of quantum computing and neuromorphic computing. History Spintronics emerged from discoveries in the 1980s concerning spin-dependent electron transport phenomena in solid-state devices. This includes the obse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Semiconductor
A semiconductor is a material which has an electrical conductivity value falling between that of a conductor, such as copper, and an insulator, such as glass. Its resistivity falls as its temperature rises; metals behave in the opposite way. Its conducting properties may be altered in useful ways by introducing impurities (" doping") into the crystal structure. When two differently doped regions exist in the same crystal, a semiconductor junction is created. The behavior of charge carriers, which include electrons, ions, and electron holes, at these junctions is the basis of diodes, transistors, and most modern electronics. Some examples of semiconductors are silicon, germanium, gallium arsenide, and elements near the so-called " metalloid staircase" on the periodic table. After silicon, gallium arsenide is the second-most common semiconductor and is used in laser diodes, solar cells, microwave-frequency integrated circuits, and others. Silicon is a critical elem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transistor
upright=1.4, gate (G), body (B), source (S) and drain (D) terminals. The gate is separated from the body by an insulating layer (pink). A transistor is a semiconductor device used to Electronic amplifier, amplify or electronic switch, switch electrical signals and electrical power, power. The transistor is one of the basic building blocks of modern electronics. It is composed of semiconductor material, usually with at least three terminals for connection to an electronic circuit. A voltage or current applied to one pair of the transistor's terminals controls the current through another pair of terminals. Because the controlled (output) power can be higher than the controlling (input) power, a transistor can amplify a signal. Some transistors are packaged individually, but many more are found embedded in integrated circuits. Austro-Hungarian physicist Julius Edgar Lilienfeld proposed the concept of a field-effect transistor in 1926, but it was not possible to actually cons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
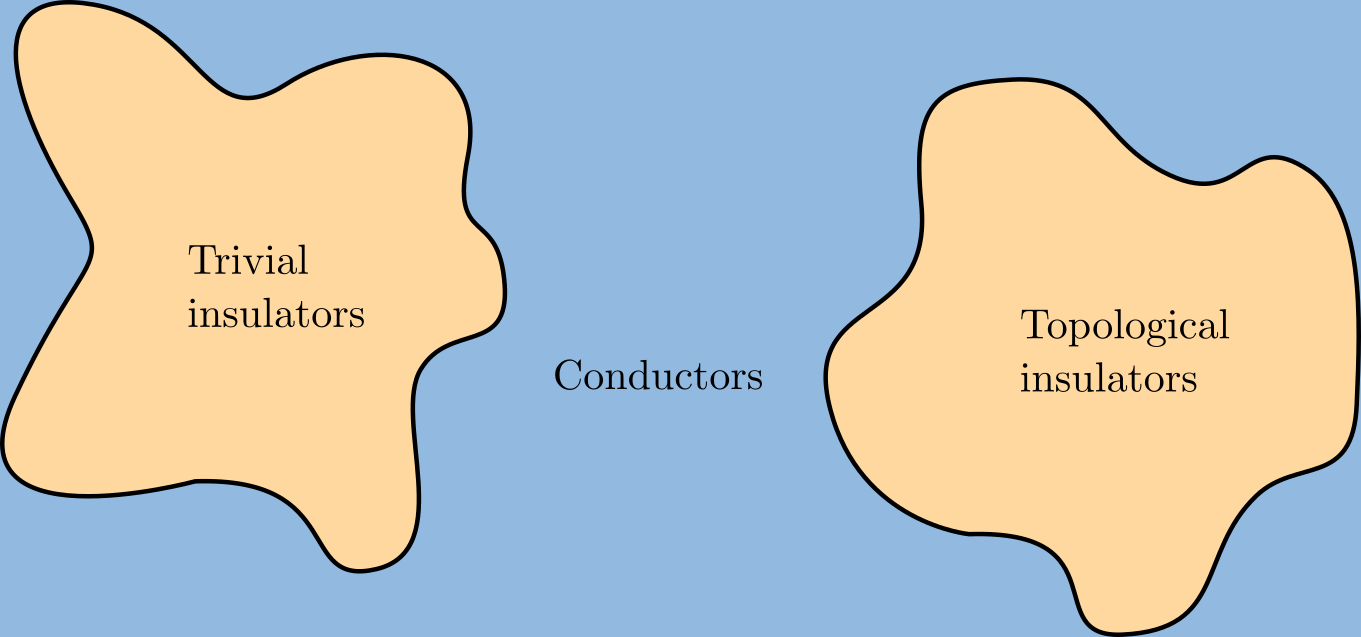
Topological Insulator
A topological insulator is a material whose interior behaves as an electrical insulator while its surface behaves as an electrical conductor, meaning that electrons can only move along the surface of the material. A topological insulator is an insulator for the same reason a "trivial" (ordinary) insulator is: there exists an energy gap between the valence and conduction bands of the material. But in a topological insulator, these bands are, in an informal sense, "twisted", relative to a trivial insulator. The topological insulator cannot be continuously transformed into a trivial one without untwisting the bands, which closes the band gap and creates a conducting state. Thus, due to the continuity of the underlying field, the border of a topological insulator with a trivial insulator (including vacuum, which is topologically trivial) is forced to support a conducting state. Since this results from a global property of the topological insulator's band structure, local (symmetr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Mobility
In solid-state physics, the electron mobility characterises how quickly an electron can move through a metal or semiconductor when pulled by an electric field. There is an analogous quantity for holes, called hole mobility. The term carrier mobility refers in general to both electron and hole mobility. Electron and hole mobility are special cases of electrical mobility of charged particles in a fluid under an applied electric field. When an electric field ''E'' is applied across a piece of material, the electrons respond by moving with an average velocity called the drift velocity, v_d. Then the electron mobility ''μ'' is defined as v_d = \mu E. Electron mobility is almost always specified in units of cm2/( V⋅ s). This is different from the SI unit of mobility, m2/( V⋅ s). They are related by 1 m2/(V⋅s) = 104 cm2/(V⋅s). Conductivity is proportional to the product of mobility and carrier concentration. For example, the same conductivity could come from a small nu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Graphene
Graphene () is an allotrope of carbon consisting of a single layer of atoms arranged in a hexagonal lattice nanostructure. "Carbon nanostructures for electromagnetic shielding applications", Mohammed Arif Poothanari, Sabu Thomas, et al., ''Industrial Applications of Nanomaterials'', 2019. "Carbon nanostructures include various low-dimensional allotropes of carbon including carbon black (CB), carbon fiber, carbon nanotubes (CNTs), fullerene, and graphene." The name is derived from "graphite" and the suffix -ene, reflecting the fact that the allotrope of carbon contains numerous double bonds. Each atom in a graphene sheet is connect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dirac Fermion
In physics, a Dirac fermion is a spin-½ particle (a fermion) which is different from its antiparticle. The vast majority of fermions – perhaps all – fall under this category. Description In particle physics, all fermions in the standard model have distinct antiparticles (''perhaps'' excepting neutrinos) and hence are Dirac fermions. They are named after Paul Dirac, and can be modeled with the Dirac equation. A Dirac fermion is equivalent to two Weyl fermions. The counterpart to a Dirac fermion is a Majorana fermion, a particle that must be its own antiparticle. Dirac quasi-particles In condensed matter physics, low-energy excitations in graphene and topological insulators, among others, are fermionic quasiparticles described by a pseudo-relativistic Dirac equation. See also * Dirac spinor, a wavefunction-like description of a Dirac fermion * Dirac–Kähler fermion, a geometric formulation of Dirac fermions * Majorana fermion A Majorana fermion (, uploaded 19 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_oxide_2.jpg)

.jpg)