|
Bennett Acceptance Ratio
The Bennett acceptance ratio method (BAR) is an algorithm for estimating the difference in free energy between two systems (usually the systems will be simulated on the computer). It was suggested by Charles H. Bennett in 1976. Preliminaries Take a system in a certain super (i.e. Gibbs) state. By performing a Metropolis Monte Carlo walk it is possible to sample the landscape of states that the system moves between, using the equation : p(\text_x \rightarrow \text_y) = \min \left(e ^ , 1 \right) = M(\beta \, \Delta U) where Δ''U'' = ''U''(State''y'') − ''U''(State''x'') is the difference in potential energy, β = 1/''kT'' (''T'' is the temperature in kelvins, while ''k'' is the Boltzmann constant), and M(x) \equiv \min(e^ , 1) is the Metropolis function. The resulting states are then sampled according to the Boltzmann distribution of the super state at temperature ''T''. Alternatively, if the system is dynamically simulated in the canonical ensemble (al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Charles H
Charles is a masculine given name predominantly found in English and French speaking countries. It is from the French form ''Charles'' of the Proto-Germanic name (in runic alphabet) or ''*karilaz'' (in Latin alphabet), whose meaning was "free man". The Old English descendant of this word was '' Ċearl'' or ''Ċeorl'', as the name of King Cearl of Mercia, that disappeared after the Norman conquest of England. The name was notably borne by Charlemagne (Charles the Great), and was at the time Latinized as ''Karolus'' (as in ''Vita Karoli Magni''), later also as '' Carolus''. Etymology The name's etymology is a Common Germanic noun ''*karilaz'' meaning "free man", which survives in English as churl (James (wikt:Appendix:Proto-Indo-European/ǵerh₂-">ĝer-, where the ĝ is a palatal consonant, meaning "to rub; to be old; grain." An old man has been worn away and is now grey with age. In some Slavic languages, the name ''Drago (given name), Drago'' (and variants: ''Drago ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
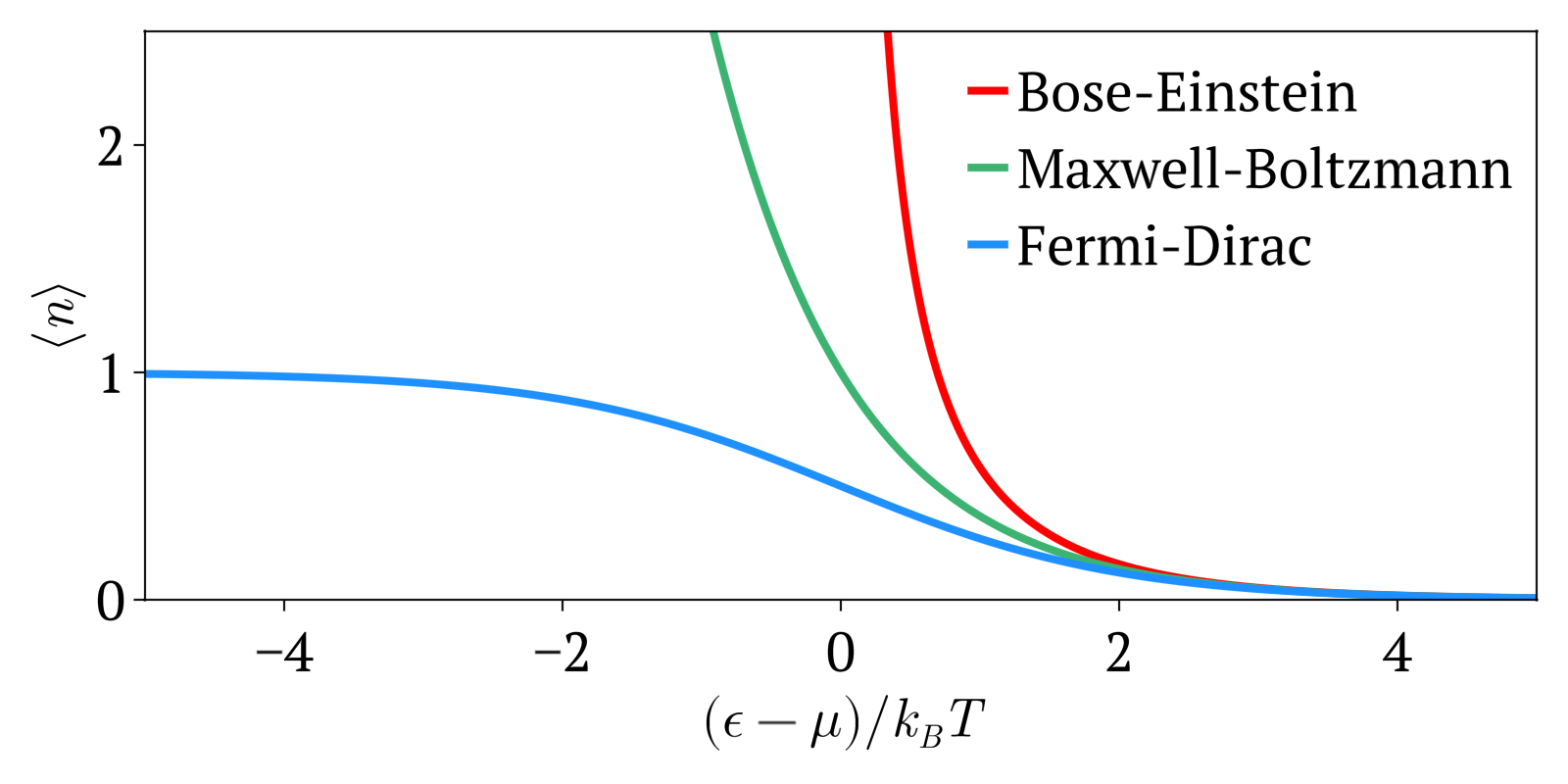
Fermi–Dirac Statistics
Fermi–Dirac statistics is a type of quantum statistics that applies to the physics of a system consisting of many non-interacting, identical particles that obey the Pauli exclusion principle. A result is the Fermi–Dirac distribution of particles over energy states. It is named after Enrico Fermi and Paul Dirac, each of whom derived the distribution independently in 1926. Fermi–Dirac statistics is a part of the field of statistical mechanics and uses the principles of quantum mechanics. Fermi–Dirac statistics applies to identical and indistinguishable particles with half-integer spin (1/2, 3/2, etc.), called fermions, in thermodynamic equilibrium. For the case of negligible interaction between particles, the system can be described in terms of single-particle energy states. A result is the Fermi–Dirac distribution of particles over these states where no two particles can occupy the same state, which has a considerable effect on the properties of the system. Fermi� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gromacs
GROMACS is a molecular dynamics package mainly designed for simulations of proteins, lipids, and nucleic acids. It was originally developed in the Biophysical Chemistry department of University of Groningen, and is now maintained by contributors in universities and research centers worldwide. GROMACS is one of the fastest and most popular software packages available, and can run on central processing units (CPUs) and graphics processing units (GPUs). It is free, open-source software released under the GNU Lesser General Public License (LGPL) ( GPL prior to Version 4.6). History The GROMACS project originally began in 1991 at Department of Biophysical Chemistry, University of Groningen, Netherlands (1991–2000). Its name originally derived from this time (GROningen MAchine for Chemical Simulations) although currently GROMACS is not an abbreviation for anything, as little active development has taken place in Groningen in recent decades. The original goal was to construct a dedica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Dynamics
Molecular dynamics (MD) is a computer simulation method for analyzing the Motion (physics), physical movements of atoms and molecules. The atoms and molecules are allowed to interact for a fixed period of time, giving a view of the dynamics (mechanics), dynamic "evolution" of the system. In the most common version, the trajectory, trajectories of atoms and molecules are determined by Numerical integration, numerically solving Newton's laws of motion, Newton's equations of motion for a system of interacting particles, where Force (physics), forces between the particles and their potential energy, potential energies are often calculated using interatomic potentials or molecular mechanics, molecular mechanical Force field (chemistry), force fields. The method is applied mostly in chemical physics, materials science, and biophysics. Because molecular systems typically consist of a vast number of particles, it is impossible to determine the properties of such complex systems analyt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Integration
Thermodynamic integration is a method used to compare the difference in Thermodynamic free energy, free energy between two given states (e.g., A and B) whose potential energies U_A and U_B have different dependences on the spatial coordinates. Because the free energy of a system is not simply a function of the phase space coordinates of the system, but is instead a function of the Boltzmann distribution, Boltzmann-weighted integral over phase space (i.e. Partition function (statistical mechanics), partition function), the free energy difference between two states cannot be calculated directly from the potential energy of just two coordinate sets (for state A and B respectively). In thermodynamic integration, the free energy difference is calculated by defining a thermodynamic path between the states and integrating over ensemble-averaged enthalpy changes along the path. Such paths can either be real chemical processes or alchemical processes. An example alchemical process is the Ki ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jensen's Inequality
In mathematics, Jensen's inequality, named after the Danish mathematician Johan Jensen, relates the value of a convex function of an integral to the integral of the convex function. It was proved by Jensen in 1906, building on an earlier proof of the same inequality for doubly-differentiable functions by Otto Hölder in 1889. Given its generality, the inequality appears in many forms depending on the context, some of which are presented below. In its simplest form the inequality states that the convex transformation of a mean is less than or equal to the mean applied after convex transformation (or equivalently, the opposite inequality for concave transformations). Jensen's inequality generalizes the statement that the secant line of a convex function lies ''above'' the graph of the function, which is Jensen's inequality for two points: the secant line consists of weighted means of the convex function (for ''t'' ∈ ,1, :t f(x_1) + (1-t) f(x_2), while the g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Free Energy Perturbation
Free-energy perturbation (FEP) is a method based on statistical mechanics that is used in computational chemistry for computing free-energy differences from molecular dynamics or Metropolis Monte Carlo simulations. The FEP method was introduced by Robert W. Zwanzig in 1954. According to the free-energy perturbation method, the free-energy difference for going from state A to state B is obtained from the following equation, known as the ''Zwanzig equation'': : \Delta F(\mathbf \to \mathbf) = F_\mathbf - F_\mathbf = -k_\text T \ln \left\langle \exp\left(-\frac \right) \right\rangle_\mathbf, where ''T'' is the temperature, ''k''B is the Boltzmann constant, and the angular brackets denote an average over a simulation run for state A. In practice, one runs a normal simulation for state A, but each time a new configuration is accepted, the energy for state B is also computed. The difference between states A and B may be in the atom types involved, in which case the Δ''F'' obtained ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Detailed Balance
The principle of detailed balance can be used in Kinetics (physics), kinetic systems which are decomposed into elementary processes (collisions, or steps, or elementary reactions). It states that at Thermodynamic equilibrium, equilibrium, each elementary process is in equilibrium with its reverse process. History The principle of detailed balance was explicitly introduced for collisions by Ludwig Boltzmann. In 1872, he proved his H-theorem using this principle.Boltzmann, L. (1964), Lectures on gas theory, Berkeley, CA, USA: U. of California Press. The arguments in favor of this property are founded upon microscopic reversibility.Richard C. Tolman, Tolman, R. C. (1938). ''The Principles of Statistical Mechanics''. Oxford University Press, London, UK. Five years before Boltzmann, James Clerk Maxwell used the principle of detailed balance for gas kinetics with the reference to the principle of sufficient reason. He compared the idea of detailed balance with other types of balancing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metropolis Monte Carlo
A metropolis () is a large city or conurbation which is a significant economic, political, and cultural area for a country or region, and an important hub for regional or international connections, commerce, and communications. A big city belonging to a larger urban agglomeration, but which is not the core of that agglomeration, is not generally considered a metropolis but a part of it. The plural of the word is ''metropolises'', although the Latin plural is , from the Greek (). For urban areas outside metropolitan areas that generate a similar attraction on a smaller scale for their region, the concept of the regiopolis ("regio" for short) was introduced by urban and regional planning researchers in Germany in 2006. Etymology () is a Greek word, (plural: ) coming from , meaning "mother" and , meaning "city" or "town", which is how the Greek colonies of antiquity referred to their original cities, with whom they retained cultic and political-cultural connections. The wo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gibbs Free Energy
In thermodynamics, the Gibbs free energy (or Gibbs energy as the recommended name; symbol is a thermodynamic potential that can be used to calculate the maximum amount of Work (thermodynamics), work, other than Work (thermodynamics)#Pressure–volume work, pressure–volume work, that may be performed by a closed system, thermodynamically closed system at constant temperature and pressure. It also provides a necessary condition for processes such as chemical reactions that may occur under these conditions. The Gibbs free energy is expressed as G(p,T) = U + pV - TS = H - TS where: * U is the internal energy of the system * H is the enthalpy of the system * S is the entropy of the system * T is the temperature of the system * V is the volume of the system * p is the pressure of the system (which must be equal to that of the surroundings for mechanical equilibrium). The Gibbs free energy change (, measured in joules in International System of Units, SI) is the ''maximum'' amount of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helmholtz Free Energy
In thermodynamics, the Helmholtz free energy (or Helmholtz energy) is a thermodynamic potential that measures the useful work obtainable from a closed thermodynamic system at a constant temperature ( isothermal). The change in the Helmholtz energy during a process is equal to the maximum amount of work that the system can perform in a thermodynamic process in which temperature is held constant. At constant temperature, the Helmholtz free energy is minimized at equilibrium. In contrast, the Gibbs free energy or free enthalpy is most commonly used as a measure of thermodynamic potential (especially in chemistry) when it is convenient for applications that occur at constant ''pressure''. For example, in explosives research Helmholtz free energy is often used, since explosive reactions by their nature induce pressure changes. It is also frequently used to define fundamental equations of state of pure substances. The concept of free energy was developed by Hermann von Helmholtz, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |