|
Atomic Emission Spectroscopy
Atomic emission spectroscopy (AES) is a method of chemical analysis that uses the intensity of light emitted from a flame, plasma, arc, or spark at a particular wavelength to determine the quantity of an element in a sample. The wavelength of the atomic spectral line in the emission spectrum gives the identity of the element while the intensity of the emitted light is proportional to the number of atoms of the element. The sample may be excited by various methods. Atomic Emission Spectroscopy allows us to measure interactions between electromagnetic radiation and physical atoms and molecules. This interaction is measured in the form of electromagnetic waves representing the changes in energy between atomic energy levels. When elements are burned by a flame, they emit electromagnetic radiation that can be recorded in the form of spectral lines. Each element has its own unique spectral line because each element has a different atomic arrangement, so this method is an important ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ICPAES PerkinElmer 2
Inductively coupled plasma atomic emission spectroscopy (ICP-AES), also referred to as inductively coupled plasma optical emission spectroscopy (ICP-OES), is an analytical technique used for the detection of chemical elements. It is a type of emission spectroscopy that uses the inductively coupled plasma to produce excited atoms and ions that emit electromagnetic radiation at wavelengths characteristic of a particular Chemical element, element. The plasma is a high temperature source of ionised source gas (often argon). The plasma is sustained and maintained by inductive coupling from electrical coils at megahertz frequencies. The source temperature is in the range from 6000 to 10,000 K. The intensity of the emissions from various wavelengths of light are proportional to the concentrations of the elements within the sample. Mechanism The ICP-AES is composed of two parts: the ICP and the optical spectrometer. The ICP torch consists of 3 concentric quartz glass tubes. The output or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flame Photometry Calcium
A flame () is the visible, gaseous part of a fire. It is caused by a highly exothermic chemical reaction made in a thin zone. When flames are hot enough to have ionized gaseous components of sufficient density, they are then considered plasma. Mechanism Color and temperature of a flame are dependent on the type of fuel involved in the combustion. For example, when a lighter is held to a candle, the applied heat causes the fuel molecules in the candle wax to vaporize. In this state they can then readily react with oxygen in the air, which gives off enough heat in the subsequent exothermic reaction to vaporize yet more fuel, thus sustaining a consistent flame. The high temperature of the flame causes the vaporized fuel molecules to decompose, forming various incomplete combustion products and free radicals, and these products then react with each other and with the oxidizer involved in the reaction of the following flame (fire). One may investigate different parts of a candle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
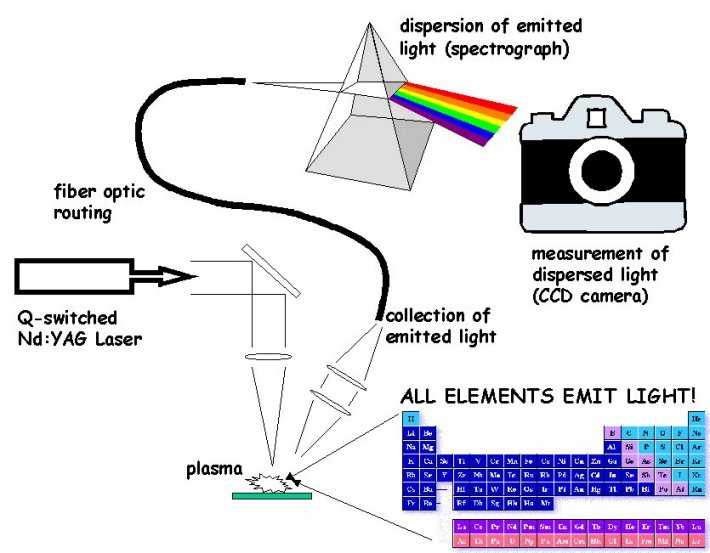
Laser-induced Breakdown Spectroscopy
Laser-induced breakdown spectroscopy (LIBS) is a type of atomic emission spectroscopy which uses a highly energetic laser pulse as the excitation source. The laser is focused to form a plasma, which atomizes and excites samples. The formation of the plasma only begins when the focused laser achieves a certain threshold for optical breakdown, which generally depends on the environment and the target material. 2000s developments From 2000 to 2010, the United States Army Research Laboratory, U.S. Army Research Laboratory (ARL) researched potential extensions to LIBS technology, which focused on hazardous material detection. Applications investigated at ARL included the standoff detection of explosive residues and other hazardous materials, plastic landmine discrimination, and material characterization of various metal alloys and polymers. Results presented by ARL suggest that LIBS may be able to discriminate between energetic and non-energetic materials. Research Broadband hig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inductively Coupled Plasma Atomic Emission Spectroscopy
Inductively coupled plasma atomic emission spectroscopy (ICP-AES), also referred to as inductively coupled plasma optical emission spectroscopy (ICP-OES), is an analytical technique used for the detection of chemical elements. It is a type of emission spectroscopy that uses the inductively coupled plasma to produce excited atoms and ions that emit electromagnetic radiation at wavelengths characteristic of a particular element. The plasma is a high temperature source of ionised source gas (often argon). The plasma is sustained and maintained by inductive coupling from electrical coils at megahertz frequencies. The source temperature is in the range from 6000 to 10,000 K. The intensity of the emissions from various wavelengths of light are proportional to the concentrations of the elements within the sample. Mechanism The ICP-AES is composed of two parts: the ICP and the optical spectrometer. The ICP torch consists of 3 concentric quartz glass tubes. The output or "work" coil of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Spectroscopy
In physics, atomic spectroscopy is the study of the electromagnetic radiation absorbed and emitted by atoms. Since unique elements have unique emission spectra, atomic spectroscopy is applied for determination of elemental compositions. It can be divided by atomization source or by the type of spectroscopy used. In the latter case, the main division is between optical and mass spectrometry. Mass spectrometry generally gives significantly better analytical performance, but is also significantly more complex. This complexity translates into higher purchase costs, higher operational costs, more operator training, and a greater number of components that can potentially fail. Because optical spectroscopy is often less expensive and has performance adequate for many tasks, it is far more common. Atomic absorption spectrometers are one of the most commonly sold and used analytical devices. Atomic spectroscopy Electrons exist in energy levels (i.e. atomic orbitals) within an atom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Absorption Spectroscopy
Atomic absorption spectroscopy (AAS) is a spectro-analytical procedure for the quantitative measurement of chemical elements. AAS is based on the absorption of light by free metallic ions that have been atomized from a sample. An alternative technique is atomic emission spectroscopy (AES). In analytical chemistry, the technique is used for determining the concentration of a particular element (the analyte) in a sample to be analyzed. AAS can be used to determine over 70 different elements in solution, or directly in solid samples via electrothermal vaporization, and is used in pharmacology, biophysics, archaeology and toxicology research. Atomic emission spectroscopy (AAS) was first used as an analytical technique, and the underlying principles were established in the second half of the 19th century by Robert Wilhelm Bunsen and Gustav Robert Kirchhoff, both professors at the University of Heidelberg, Germany. The modern form of AAS was largely developed during the 1950s by a t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Qualitative Data
Qualitative properties are properties that are observed and can generally not be measured with a numerical result, unlike Quantitative property, quantitative properties, which have numerical characteristics. Description Qualitative properties are properties that are observed and can generally not be measured with a numerical result. They are contrasted to Quantitative property, quantitative properties which have numerical characteristics. Evaluation Although measuring something in qualitative terms is difficult, most people can (and will) make a judgement about a behaviour on the basis of how they feel treated. This indicates that qualitative properties are closely related to emotional impressions. A test method can result in qualitative data about something. This can be a categorical result or a binary classification (e.g., pass/fail, go/no go, Conformity, conform/non-conform). It can sometimes be an engineering judgement. Categorization The data that all share a qualitat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conductive
In physics and electrical engineering, a conductor is an object or type of material that allows the flow of Electric charge, charge (electric current) in one or more directions. Materials made of metal are common electrical conductors. The flow of negatively charged electrons generates electric current, positively charged Electron hole, holes, and positive or negative ions in some cases. In order for current to flow within a closed Electrical network, electrical circuit, one charged particle does not need to travel from the component producing the current (the current source) to those consuming it (the Electrical load, loads). Instead, the charged particle simply needs to nudge its neighbor a finite amount, who will nudge ''its'' neighbor, and on and on until a particle is nudged into the consumer, thus powering it. Essentially what is occurring is a long chain of momentum transfer between mobile charge carriers; the Drude model of conduction describes this process more rigorous ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on a large scale (1.3million metric tons per year in 2022) for uses in many critical industries including refractories (50%), lithium-ion batteries (18%), foundries (10%), and lubricants (5%), among others (17%). Graphite converts to diamond under extremely high pressure and temperature. Graphite's low cost, thermal and chemical inertness and characteristic conductivity of heat and electricity finds numerous applications in high energy and high temperature processes. Types and varieties Graphite can occur naturally or be produced synthetically. Natural graphite is obtained from naturally occurring geologic deposits and synthetic graphite is produced t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrostatic Discharge
Electrostatic discharge (ESD) is a sudden and momentary flow of electric current between two differently-charged objects when brought close together or when the dielectric between them breaks down, often creating a visible electric spark, spark associated with the static electricity between the objects. ESD can create spectacular electric sparks (lightning, with the accompanying sound of thunder, is an example of a large-scale ESD event), but also less dramatic forms, which may be neither seen nor heard, yet still be large enough to cause damage to sensitive electronic devices. Electric sparks require a field strength above approximately 4 million V/m in air, as notably occurs in lightning strikes. Other forms of ESD include corona discharge from sharp electrodes, brush discharge from blunt electrodes, etc. ESD can cause harmful effects of importance in industry, including explosions in gas, fuel vapor and coal dust, as well as failure of solid state electronics components such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Induction Coil
An induction coil or "spark coil" ( archaically known as an inductorium or Ruhmkorff coil after Heinrich Rühmkorff) is a type of transformer used to produce high-voltage pulses from a low-voltage direct current (DC) supply. p.98 To create the flux changes necessary to induce voltage in the secondary coil, the direct current in the primary coil is repeatedly interrupted by a vibrating mechanical contact called an interrupter. Invented in 1836 by the Irish-Catholic priest Nicholas Callan, also independently by American inventor Charles Grafton Page, the induction coil was the first type of transformer. It was widely used in x-ray machines, spark-gap radio transmitters, arc lighting and quack medical electrotherapy devices from the 1880s to the 1920s. Today its only common use is as the ignition coils in internal combustion engines and in physics education to demonstrate induction. Construction and function An induction coil consists of two coils of insulated wire wo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |