|
Arthrobacter Bussei
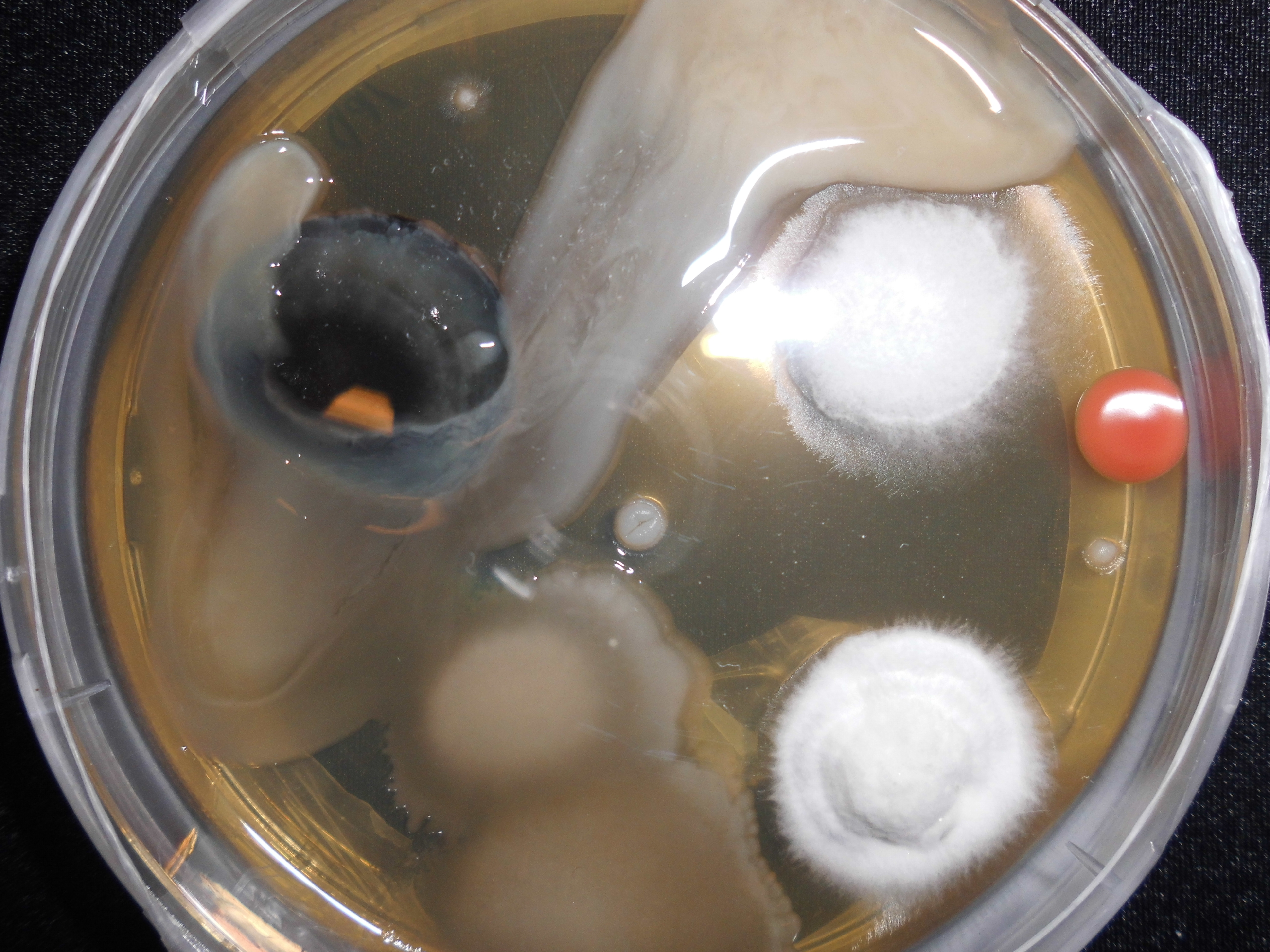
''Arthrobacter bussei'' (bus’se.i. N.L. gen. n. ''bussei'', of Busse; named after the German microbiologist Hans-Jürgen Busse) is a pink-coloured, aerobic, coccus-shaped, Gram-stain-positive, oxidase-positive and catalase-positive bacterium isolated from cheese made of cow´s milk. ''A. bussei'' is non-motile and does not form spores. Rod–coccus life cycle is not observed. Cells are 1.1–1.5 µm in diameter. On trypticase soy agar it forms pink-coloured, raised and round colonies, which are 1.0 mm in diameter after 5 days at 30 °C The genome of the strain ''A. bussei'' KR32T has been fully sequenced. Characteristics Morphology The cells of ''A. bussei'' are coccus-shaped. The bacterium is Gram-stain-positive. The cells have a diameter of 1.1–1.5 μm. A rod-coccus life cycle is not observed. The species is not flagellated and therefore non-motile. Endospores are not formed. On trypticase soy agar the cells forms very small colonies. Their diameter is 1.0 mm after ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coccus
A coccus (plural cocci) is any bacterium or archaeon that has a spherical, ovoid, or generally round shape. Bacteria are categorized based on their shapes into three classes: cocci (spherical-shaped), bacillus (rod-shaped) and spiral ( of which there are two types: spirillum and spirochete). Coccus refers to the shape of the bacteria, and can contain multiple genera, such as staphylococci or streptococci. Cocci can grow in pairs, chains, or clusters, depending on their orientation and attachment during cell division. In contrast to many bacilli-shaped bacteria, most cocci bacteria do not have flagella and are non-motile. Cocci is an English loanword of a modern or neo-Latin noun, which in turn stems from the Greek masculine noun () meaning 'berry'. Structure The cell wall structure for cocci may vary between gram-positive (thick peptidoglycan layers) and gram-negative (thin peptidoglycan layers). While living in their host organism, cocci can be pathogenic (e.g., strept ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agar Plate
An agar plate is a Petri dish that contains a growth medium solidified with agar, used to culture microorganisms. Sometimes selective compounds are added to influence growth, such as antibiotics. Individual microorganisms placed on the plate will grow into individual colonies, each a clone genetically identical to the individual ancestor organism (except for the low, unavoidable rate of mutation). Thus, the plate can be used either to estimate the concentration of organisms in a liquid culture or a suitable dilution of that culture using a colony counter, or to generate genetically pure cultures from a mixed culture of genetically different organisms. Several methods are available to plate out cells. One technique is known as " streaking". In this technique, a drop of the culture on the end of a thin, sterile loop of wire, sometimes known as an inoculator, is streaked across the surface of the agar leaving organisms behind, a higher number at the beginning of the streak and a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta-galactosidase
β-Galactosidase (EC 3.2.1.23, lactase, beta-gal or β-gal; systematic name β-D-galactoside galactohydrolase), is a glycoside hydrolase enzyme that catalyzes hydrolysis of terminal non-reducing β-D-galactose residues in β-D-galactosides. β-Galactosides include carbohydrates containing galactose where the glycosidic bond lies above the galactose molecule. Substrates of different β-galactosidases include ganglioside GM1, lactosylceramides, lactose, and various glycoproteins. Function β-Galactosidase is an exoglycosidase which hydrolyzes the β-glycosidic bond formed between a galactose and its organic moiety. It may also cleave fucosides and arabinosides but with much lower efficiency. It is an essential enzyme in the human body. Deficiencies in the protein can result in galactosialidosis or Morquio B syndrome. In '' E. coli'', the ''lacZ'' gene is the structural gene for β-galactosidase; which is present as part of the inducible system ''lac'' operon which is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha-galactosidase
α-Galactosidase ( EC 3.2.1.22, α-GAL, α-GAL A; systematic name α-D-galactoside galactohydrolase) is a glycoside hydrolase enzyme that catalyses the following reaction: : Hydrolysis of terminal, non-reducing α-D-galactose residues in α-D-galactosides, including galactose oligosaccharides, galactomannans and galactolipids It catalyzes many catabolic processes, including cleavage of glycoproteins, glycolipids, and polysaccharides. The enzyme is encoded by the ''GLA'' gene. Two recombinant forms of human α-galactosidase are called agalsidase α (INN) and agalsidase β (INN). A mold-derived form is the primary ingredient in gas relief supplements. Function This enzyme is a homodimeric glycoprotein that hydrolyses the terminal α-galactosyl moieties from glycolipids and glycoproteins. It predominantly hydrolyzes ceramide trihexoside, and it can catalyze the hydrolysis of melibiose into galactose and glucose. Reaction mechanism Disease relevance Fabry disease ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trypsin
Trypsin is an enzyme in the first section of the small intestine that starts the digestion of protein molecules by cutting these long chains of amino acids into smaller pieces. It is a serine protease from the PA clan superfamily, found in the digestive system of many vertebrates, where it hydrolyzes proteins. Trypsin is formed in the small intestine when its proenzyme form, the trypsinogen produced by the pancreas, is activated. Trypsin cuts peptide chains mainly at the carboxyl side of the amino acids lysine or arginine. It is used for numerous biotechnological processes. The process is commonly referred to as trypsin proteolysis or trypsinization, and proteins that have been digested/treated with trypsin are said to have been trypsinized. Trypsin was discovered in 1876 by Wilhelm Kühne and was named from the Ancient Greek word for rubbing since it was first isolated by rubbing the pancreas with glycerin. Function In the duodenum, trypsin catalyzes the hydro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cystine
Cystine is the oxidized derivative of the amino acid cysteine and has the formula (SCH2CH(NH2)CO2H)2. It is a white solid that is poorly soluble in water. As a residue in proteins, cystine serves two functions: a site of redox reactions and a mechanical linkage that allows proteins to retain their three-dimensional structure. Formation and reactions Structure Cystine is the disulfide derived from the amino acid cysteine. The conversion can be viewed as an oxidation: : Cystine contains a disulfide bond, two amine groups, and two carboxylic acid groups. As for other amino acids, the amine and carboxylic acid groups exist is rapid equilibrium with the ammonium-carboxylate tautomer. The great majority of the literature concerns the ''l,l-''cystine, derived from ''l''-cysteine. Other isomers include ''d,d''-cystine and the meso isomer d,l-cystine, neither of which is biologically significant. Occurrence Cystine is common in many foods such as eggs, meat, dairy products, and w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valine
Valine (symbol Val or V) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α- amino group (which is in the protonated −NH3+ form under biological conditions), an α- carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain isopropyl group, making it a non-polar aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Human dietary sources are foods that contain protein, such as meats, dairy products, soy products, beans and legumes. It is encoded by all codons starting with GU (GUU, GUC, GUA, and GUG). History and etymology Valine was first isolated from casein in 1901 by Hermann Emil Fischer. The name valine comes from valeric acid, which in turn is named after the plant valerian due to the presence of the acid in the roots of the plant. Nomenclature According to IUPAC, carbon atoms forming valine are numbered sequenti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arylamidase
Cytosol alanyl aminopeptidase (, ''arylamidase'', ''aminopolypeptidase'', ''thiol-activated aminopeptidase'', ''human liver aminopeptidase'', ''puromycin-sensitive aminopeptidase'', ''soluble alanyl aminopeptidase'', ''cytosol aminopeptidase III'', ''alanine aminopeptidase'') is an enzyme. This enzyme catalyses the release of an N-terminal amino acid, preferentially alanine, from a wide range of peptides, amides and arylamides. This puromycin-sensitive enzyme is a Co2+-activated zinc-sialoglycoprotein A sialoglycoprotein is a combination of sialic acid and glycoprotein, which is, itself, a combination of sugar and protein. These proteins often contain one or more sialyl oligosaccharides that are covalently bound to the rest of the molecule. .... References External links * {{Portal bar, Biology, border=no EC 3.4.11 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leucine
Leucine (symbol Leu or L) is an essential amino acid that is used in the biosynthesis of proteins. Leucine is an α-amino acid, meaning it contains an α- amino group (which is in the protonated −NH3+ form under biological conditions), an α- carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain isobutyl group, making it a non-polar aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Human dietary sources are foods that contain protein, such as meats, dairy products, soy products, and beans and other legumes. It is encoded by the codons UUA, UUG, CUU, CUC, CUA, and CUG. Like valine and isoleucine, leucine is a branched-chain amino acid. The primary metabolic end products of leucine metabolism are acetyl-CoA and acetoacetate; consequently, it is one of the two exclusively ketogenic amino acids, with lysine being the other. It is the most imp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipase
Lipase ( ) is a family of enzymes that catalyzes the hydrolysis of fats. Some lipases display broad substrate scope including esters of cholesterol, phospholipids, and of lipid-soluble vitamins and sphingomyelinases; however, these are usually treated separately from "conventional" lipases. Unlike esterases, which function in water, lipases "are activated only when adsorbed to an oil–water interface". Lipases perform essential roles in digestion, transport and processing of dietary lipids in most, if not all, organisms. Structure and catalytic mechanism Classically, lipases catalyse the hydrolysis of triglycerides: :triglyceride + H2O → fatty acid + diacylglycerol :diacylglycerol + H2O → fatty acid + monacylglycerol :monacylglycerol + H2O → fatty acid + glycerol Lipases are serine hydrolases, i.e. they function by transesterification generating an acyl serine intermediate. Most lipases act at a specific position on the glycerol backbone of a lipid subs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Esterase
An esterase is a hydrolase enzyme that splits esters into an acid and an alcohol in a chemical reaction with water called hydrolysis. A wide range of different esterases exist that differ in their substrate specificity, their protein structure, and their biological function. EC classification/list of enzymes * ''EC 3.1.1'': Carboxylic ester hydrolases ** Acetylesterase (EC 3.1.1.6), splits off acetyl groups *** Cholinesterase **** Acetylcholinesterase, inactivates the neurotransmitter acetylcholine **** Pseudocholinesterase, broad substrate specificity, found in the blood plasma and in the liver ** Pectinesterase (EC 3.1.1.11), clarifies fruit juices * ''EC 3.1.2'': Thiolester hydrolases ** Thioesterase *** Ubiquitin carboxy-terminal hydrolase L1 * ''EC 3.1.3'': Phosphoric monoester hydrolases ** Phosphatase (EC 3.1.3.x), hydrolyses phosphoric acid monoesters into a phosphate ion and an alcohol *** Alkaline phosphatase, removes phosphate groups from many types ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaline Phosphatase
The enzyme alkaline phosphatase (EC 3.1.3.1, alkaline phosphomonoesterase; phosphomonoesterase; glycerophosphatase; alkaline phosphohydrolase; alkaline phenyl phosphatase; orthophosphoric-monoester phosphohydrolase (alkaline optimum), systematic name phosphate-monoester phosphohydrolase (alkaline optimum)) catalyses the following reaction: : a phosphate monoester + H2O = an alcohol + phosphate Alkaline phosphatase has the physiological role of dephosphorylating compounds. The enzyme is found across a multitude of organisms, prokaryotes and eukaryotes alike, with the same general function but in different structural forms suitable to the environment they function in. Alkaline phosphatase is found in the periplasmic space of '' E. coli'' bacteria. This enzyme is heat stable and has its maximum activity at high pH. In humans, it is found in many forms depending on its origin within the body – it plays an integral role in metabolism within the liver and development withi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |