|
Actinides
The actinide () or actinoid () series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. Number 103, lawrencium, is also generally included despite being part of the 6d transition series. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide. The 1985 IUPAC ''Red Book'' recommends that ''actinoid'' be used rather than ''actinide'', since the suffix ''-ide'' normally indicates a negative ion. However, owing to widespread current use, ''actinide'' is still allowed. Actinium through nobelium are f-block elements, while lawrencium is a d-block element and a transition metal. The series mostly corresponds to the filling of the 5f electron shell, although as isolated atoms in the ground state many have anomalous configurations involving the filling of the 6d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Americium
Americium is a synthetic element, synthetic chemical element; it has Chemical symbol, symbol Am and atomic number 95. It is radioactive and a transuranic member of the actinide series in the periodic table, located under the lanthanide element europium and was thus named after the Americas by analogy. Americium was first produced in 1944 by the group of Glenn T. Seaborg from Berkeley, California, at the Metallurgical Laboratory of the University of Chicago, as part of the Manhattan Project. Although it is the third element in the transuranic series, it was discovered fourth, after the heavier curium. The discovery was kept secret and only released to the public in November 1945. Most americium is produced by uranium or plutonium being bombarded with neutrons in nuclear reactors – one tonne of spent nuclear fuel contains about 100 grams of americium. It is widely used in commercial ionization chamber smoke detectors, as well as in neutron sources and industrial gauges. Several u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neptunium
Neptunium is a chemical element; it has chemical symbol, symbol Np and atomic number 93. A radioactivity, radioactive actinide metal, neptunium is the first transuranic element. It is named after Neptune, the planet beyond Uranus in the Solar System, which uranium is named after. A neptunium atom has 93 protons and 93 electrons, of which seven are valence electrons. Neptunium metal is silvery and tarnishes when exposed to air. The element occurs in three allotrope, allotropic forms and it normally exhibits five oxidation states, ranging from +3 to +7. Like all actinides, it is Radioactive decay, radioactive, radiation poisoning, poisonous, pyrophoricity, pyrophoric, and capable of accumulating in bones, which makes the handling of neptunium dangerous. Although many false claims of its discovery were made over the years, the element was first synthesized by Edwin McMillan and Philip H. Abelson at the Berkeley Radiation Laboratory in 1940. Since then, most neptunium has been and still ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
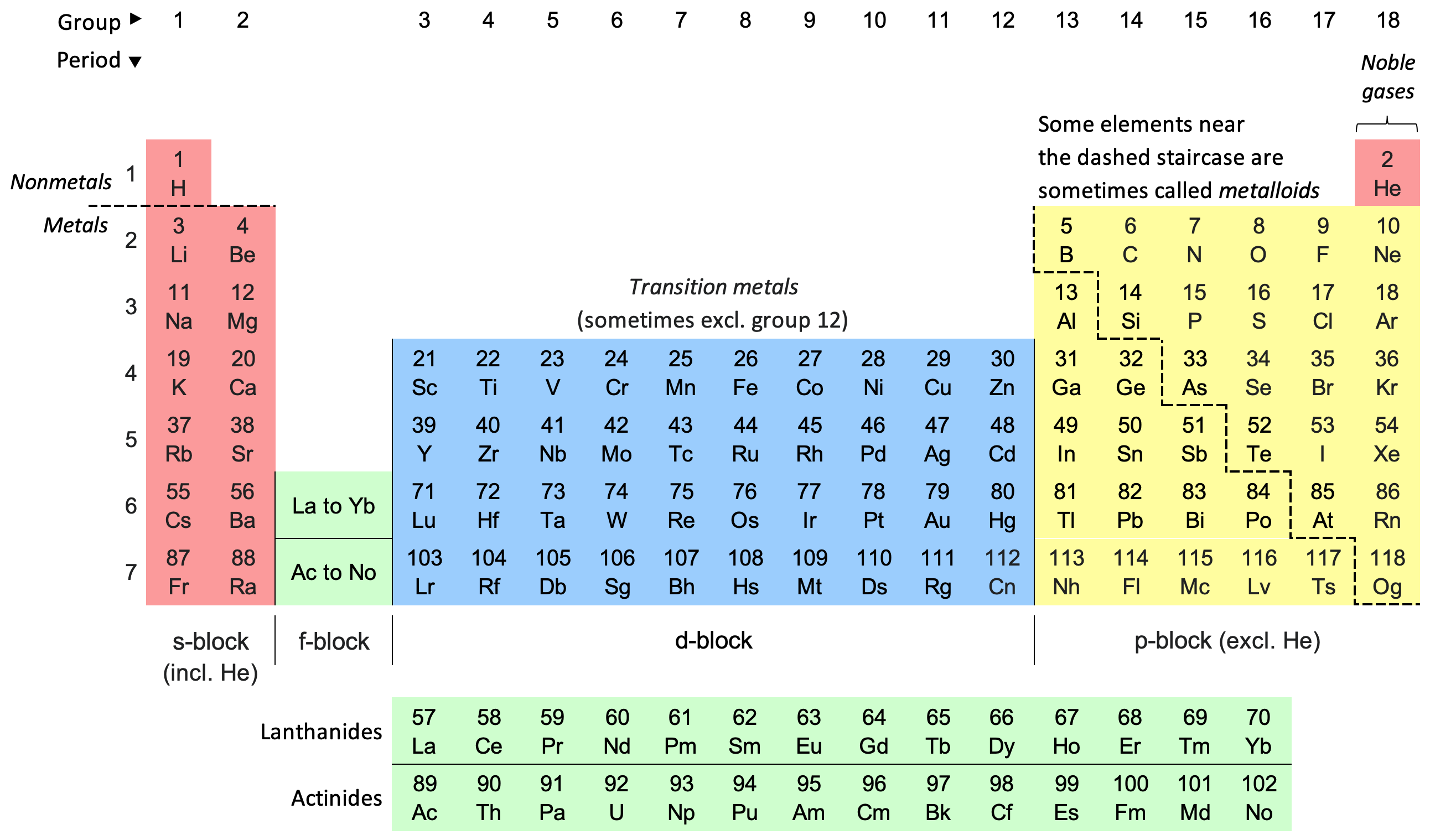
Periodic Table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other sciences. It is a depiction of the periodic law, which states that when the elements are arranged in order of their atomic numbers an approximate recurrence of their properties is evident. The table is divided into four roughly rectangular areas called blocks. Elements in the same group tend to show similar chemical characteristics. Vertical, horizontal and diagonal trends characterize the periodic table. Metallic character increases going down a group and from right to left across a period. Nonmetallic character increases going from the bottom left of the periodic table to the top right. The first periodic table to become generally accepted was that of the Russian chemist Dmitri Mendeleev in 1869; he formulated the periodic law as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Curium
Curium is a synthetic chemical element; it has symbol Cm and atomic number 96. This transuranic actinide element was named after eminent scientists Marie and Pierre Curie, both known for their research on radioactivity. Curium was first intentionally made by the team of Glenn T. Seaborg, Ralph A. James, and Albert Ghiorso in 1944, using the cyclotron at Berkeley. They bombarded the newly discovered element plutonium (the isotope 239Pu) with alpha particles. This was then sent to the Metallurgical Laboratory at University of Chicago where a tiny sample of curium was eventually separated and identified. The discovery was kept secret until after the end of World War II. The news was released to the public in November 1947. Most curium is produced by bombarding uranium or plutonium with neutrons in nuclear reactors – one tonne of spent nuclear fuel contains ~20 grams of curium. Curium is a hard, dense, silvery metal with a high melting and boiling point for an actinide. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nobelium
Nobelium is a synthetic element, synthetic chemical element; it has Chemical symbol, symbol No and atomic number 102. It is named after Alfred Nobel, the inventor of dynamite and benefactor of science. A radioactive metal, it is the tenth transuranium element, the second transfermium, and is the penultimate member of the actinide series. Like all elements with atomic number over 100, nobelium can only be produced in particle accelerators by bombarding lighter elements with charged particles. A total of twelve isotopes of nobelium, nobelium isotopes are known to exist; the most stable is 259No with a half-life of 58 minutes, but the shorter-lived 255No (half-life 3.1 minutes) is most commonly used in chemistry because it can be produced on a larger scale. Chemistry experiments have confirmed that nobelium behaves as a heavier Homologous series, homolog to ytterbium in the periodic table. The chemical properties of nobelium are not completely known: they are mostly only known in aque ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lawrencium
Lawrencium is a synthetic chemical element; it has symbol Lr (formerly Lw) and atomic number 103. It is named after Ernest Lawrence, inventor of the cyclotron, a device that was used to discover many artificial radioactive elements. A radioactive metal, lawrencium is the eleventh transuranium element, the third transfermium, and the last member of the actinide series. Like all elements with atomic number over 100, lawrencium can only be produced in particle accelerators by bombarding lighter elements with charged particles. Fourteen isotopes of lawrencium are currently known; the most stable is 266Lr with half-life 11 hours, but the shorter-lived 260Lr (half-life 2.7 minutes) is most commonly used in chemistry because it can be produced on a larger scale. Chemistry experiments confirm that lawrencium behaves as a heavier homolog to lutetium in the periodic table, and is a trivalent element. It thus could also be classified as the first of the 7th-period transition metals. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium (element 71) is also sometimes considered a lanthanide, despite being a d-block element and a transition metal. The informal chemical symbol Ln is used in general discussions of lanthanide chemistry to refer to any lanthanide. All but one of the lanthanides are f-block elements, corresponding to the filling of the 4f electron shell. Lutetium is a d-block element (thus also a transition metal), and on this basis its inclusion has been questioned; however, like its congeners scandium and yttrium in group 3, it behaves similarly to the other 14. The term rare-earth element or rare-earth metal is often used to include the stable group 3 elements Sc, Y, and Lu in addition to the 4f elements. All lanthanide elements form trivalent cations, Ln3+, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered ''radioactive''. Three of the most common types of decay are Alpha decay, alpha, Beta decay, beta, and Gamma ray, gamma decay. The weak force is the Fundamental interactions, mechanism that is responsible for beta decay, while the other two are governed by the electromagnetic force, electromagnetic and nuclear forces. Radioactive decay is a randomness, random process at the level of single atoms. According to quantum mechanics, quantum theory, it is impossible to predict when a particular atom will decay, regardless of how long the atom has existed. However, for a significant number of identical atoms, the overall decay rate can be expressed as a decay constant or as a half-life. The half-lives of radioactive atoms have a huge range: f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium radioactive decay, radioactively decays, usually by emitting an alpha particle. The half-life of this decay varies between 159,200 and 4.5 billion years for different isotopes of uranium, isotopes, making them useful for dating the age of the Earth. The most common isotopes in natural uranium are uranium-238 (which has 146 neutrons and accounts for over 99% of uranium on Earth) and uranium-235 (which has 143 neutrons). Uranium has the highest atomic weight of the primordial nuclide, primordially occurring elements. Its density is about 70% higher than that of lead and slightly lower than that of gold or tungsten. It occurs naturally in low concentrations of a few Parts-per notation#Parts-per expressions, parts per million in soil, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plutonium
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation states. It reacts with carbon, halogens, nitrogen, silicon, and hydrogen. When exposed to moist air, it forms oxides and hydrides that can expand the sample up to 70% in volume, which in turn flake off as a powder that is pyrophoric. It is radioactive and can accumulate in bones, which makes the handling of plutonium dangerous. Plutonium was first synthesized and isolated in late 1940 and early 1941, by deuteron bombardment of uranium-238 in the cyclotron at the University of California, Berkeley. First, neptunium-238 (half-life 2.1 days) was synthesized, which then beta-decayed to form the new element with atomic number 94 and atomic weight 238 (half-life 88 years). Since uranium had been named after the planet Uranus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thorium
Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive grey when it is exposed to air, forming thorium dioxide; it is moderately soft, malleable, and has a high melting point. Thorium is an electropositive actinide whose chemistry is dominated by the +4 oxidation state; it is quite reactive and can ignite in air when finely divided. All known thorium isotopes are unstable. The most stable isotope, 232Th, has a half-life of 14.05 billion years, or about the age of the universe; it decays very slowly via alpha decay, starting a decay chain named the thorium series that ends at stable 208 Pb. On Earth, thorium and uranium are the only elements with no stable or nearly-stable isotopes that still occur naturally in large quantities as primordial elements. Thorium is estimated to be over three times as abundant as uranium in the Earth's crust, and is chiefly refined from monazite sa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protactinium
Protactinium is a chemical element; it has symbol Pa and atomic number 91. It is a dense, radioactive, silvery-gray actinide metal which readily reacts with oxygen, water vapor, and inorganic acids. It forms various chemical compounds, in which protactinium is usually present in the oxidation state +5, but it can also assume +4 and even +3 or +2 states. Concentrations of protactinium in the Earth's crust are typically a few parts per trillion, but may reach up to a few parts per million in some uraninite ore deposits. Because of its scarcity, high radioactivity, and high toxicity, there are currently no uses for protactinium outside scientific research, and for this purpose, protactinium is mostly extracted from spent nuclear fuel. The element was first identified in 1913 by Kazimierz Fajans and Oswald Helmuth Göhring and named "brevium" because of the short half-life of the specific isotope studied, 234mPa. A more stable isotope of protactinium, 231Pa, was discovered in 191 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |