|
2-aminoisobutyric Acid
2-Aminoisobutyric acid (also known as α-aminoisobutyric acid, AIB, α-methylalanine, or 2-methylalanine) is the non-proteinogenic amino acid with the structural formula H2N-C(CH3)2-COOH. It is rare in nature, having been only found in meteorites, and some antibiotics of fungal origin, such as alamethicin and some lantibiotics. Synthesis In the laboratory, 2-aminoisobutyric acid may be prepared from acetone cyanohydrin, by reaction with ammonia followed by hydrolysis. Industrial scale synthesis can be achieved by the selective hydroamination of methacrylic acid. Biological activity 2-Aminoisobutyric acid is not one of the proteinogenic amino acids and is rather rare in nature (''cf.'' non-proteinogenic amino acids). It is a strong helix inducer in peptides due to Thorpe–Ingold effect of its gem-dimethyl group. Oligomers of AIB form 310 helices. Ribosomal incorporation into peptides 2-Aminoisobutyric acid is compatible with ribosomal elongation of peptide synthesis. K ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CRC Press
The CRC Press, LLC is an American publishing group that specializes in producing technical books. Many of their books relate to engineering, science and mathematics. Their scope also includes books on business, forensics and information technology. CRC Press is now a division of Taylor & Francis, itself a subsidiary of Informa. History The CRC Press was founded as the Chemical Rubber Company (CRC) in 1903 by brothers Arthur, Leo and Emanuel Friedman in Cleveland, Ohio, based on an earlier enterprise by Arthur, who had begun selling rubber laboratory aprons in 1900. The company gradually expanded to include sales of laboratory equipment to chemists. In 1913 the CRC offered a short (116-page) manual called the ''Rubber Handbook'' as an incentive for any purchase of a dozen aprons. Since then the ''Rubber Handbook'' has evolved into the CRC's flagship book, the ''CRC Handbook of Chemistry and Physics''. In 1964, Chemical Rubber decided to focus on its publishing ventures, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-proteinogenic Amino Acids
In biochemistry, non-coded or non-proteinogenic amino acids are distinct from the 22 proteinogenic amino acids (21 in eukaryotesplus formylmethionine in eukaryotes with prokaryote organelles like mitochondria) which are naturally encoded in the genome of organisms for the assembly of proteins. However, over 140 non-proteinogenic amino acids occur naturally in proteins and thousands more may occur in nature or be synthesized in the laboratory. Chemically synthesized amino acids can be called unnatural amino acids. Unnatural amino acids can be synthetically prepared from their native analogs via modifications such as amine alkylation, side chain substitution, structural bond extension cyclization, and isosteric replacements within the amino acid backbone. Many non-proteinogenic amino acids are important: * intermediates in biosynthesis, * in post-translational formation of proteins, * in a physiological role (e.g. components of bacterial cell walls, neurotransmitters and toxins), * n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Branched-chain Amino Acids
A branched-chain amino acid (BCAA) is an amino acid having an aliphatic side-chain with a branch (a central carbon atom bound to three or more carbon atoms). Among the proteinogenic amino acids, there are three BCAAs: leucine, isoleucine, and valine. Non-proteinogenic BCAAs include 2-aminoisobutyric acid. The three proteinogenic BCAAs are among the nine essential amino acids for humans, accounting for 35% of the essential amino acids in muscle proteins and 40% of the preformed amino acids required by mammals. Synthesis for BCAAs occurs in all locations of plants, within the plastids of the cell, as determined by presence of mRNAs which encode for enzymes in the metabolic pathway. BCAAs fill several metabolic and physiologic roles. Metabolically, BCAAs promote protein synthesis and turnover, signaling pathways, and metabolism of glucose. Oxidation of BCAAs may increase fatty acid oxidation and play a role in obesity. Physiologically, BCAAs take on roles in the immune system ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valine—tRNA Ligase
In enzymology, a valine—tRNA ligase () is an enzyme that catalyzes the chemical reaction :ATP + L-valine + tRNAVal \rightleftharpoons AMP + diphosphate + L-valyl-tRNAVal The 3 substrates of this enzyme are ATP, L-valine, and tRNA(Val), whereas its 3 products are AMP, diphosphate, and L-valyl-tRNA(Val). This enzyme belongs to the family of ligases, to be specific those forming carbon-oxygen bonds in aminoacyl-tRNA and related compounds. The systematic name of this enzyme class is L-valine:tRNAVal ligase (AMP-forming). Other names in common use include valyl-tRNA synthetase, valyl-transfer ribonucleate synthetase, valyl-transfer RNA synthetase, valyl-transfer ribonucleic acid synthetase, valine transfer ribonucleate ligase, and valine translase. This enzyme participates in valine, leucine and isoleucine biosynthesis and aminoacyl-trna biosynthesis. Structural studies As of late 2007, 5 structures A structure is an arrangement and organization of interrelated elemen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elongation Factor P
EF-P (elongation factor P) is an essential protein that in bacteria stimulates the formation of the first peptide bonds in protein synthesis. Studies show that EF-P prevents ribosomes from stalling during the synthesis of proteins containing consecutive prolines. EF-P binds to a site located between the binding site for the peptidyl tRNA ( P site) and the exiting tRNA ( E site). It spans both ribosomal subunits with its amino-terminal domain positioned adjacent to the aminoacyl acceptor stem and its carboxyl-terminal domain positioned next to the anticodon stem-loop of the P site-bound initiator tRNA. The EF-P protein shape and size is very similar to a tRNA and interacts with the ribosome via the exit “E” site on the 30S subunit and the peptidyl-transferase center (PTC) of the 50S subunit. EF-P is a translation aspect of an unknown function, therefore It probably functions indirectly by altering the affinity of the ribosome for aminoacyl-tRNA, thus increasing their reactivi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flexizyme
An expanded genetic code is an artificially modified genetic code in which one or more specific codons have been re-allocated to encode an amino acid that is not among the 22 common naturally-encoded proteinogenic amino acids. The key prerequisites to expand the genetic code are: * the non-standard amino acid to encode, * an unused codon to adopt, * a tRNA that recognises this codon, and * a tRNA synthetase that recognises only that tRNA and only the non-standard amino acid. Expanding the genetic code is an area of research of synthetic biology, an applied biological discipline whose goal is to engineer living systems for useful purposes. The genetic code expansion enriches the repertoire of useful tools available to science. In May 2019, researchers, in a milestone effort, reported the creation of a new synthetic (possibly artificial) form of viable life, a variant of the bacteria ''Escherichia coli'', by reducing the natural number of 64 codons in the bacterial genome to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
310 Helix
A 310 helix is a type of secondary structure found in proteins and polypeptides. Of the numerous protein secondary structures present, the 310-helix is the fourth most common type observed; following α-helices, β-sheets and reverse turns. 310-helices constitute nearly 10–15% of all helices in protein secondary structures, and are typically observed as extensions of α-helices found at either their N- or C- termini. Because of the α-helices tendency to consistently fold and unfold, it has been proposed that the 310-helix serves as an intermediary conformation of sorts, and provides insight into the initiation of α-helix folding. Discovery Max Perutz, the head of the Medical Research Council Laboratory of Molecular Biology at the University of Cambridge, wrote the first paper documenting the elusive 310-helix. Together with Lawrence Bragg and John Kendrew, Perutz published an exploration of polypeptide chain configurations in 1950, based on cues from noncrystalline dif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Group
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bounded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For example, protonation of methanol gives an e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geminal
In chemistry, the descriptor geminal () refers to the relationship between two atoms or functional groups that are attached to the same atom. A geminal diol, for example, is a diol (a molecule that has two alcohol functional groups) attached to the same carbon atom, as in methanediol. Also the shortened prefix ''gem'' may be applied to a chemical name to denote this relationship, as in a ''gem''-dibromide for "geminal dibromide". The concept is important in many branches of chemistry, including synthesis and spectroscopy, because functional groups attached to the same atom often behave differently from when they are separated. Geminal diols, for example, are easily converted to ketones or aldehydes with loss of water.Peter Taylor (2002)''Mechanism and synthesis'' Book 10 of ''Molecular world''. Open University, Royal Society of Chemistry; . 368 pages The related term vicinal refers to the relationship between two functional groups that are attached to adjacent atoms. The re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
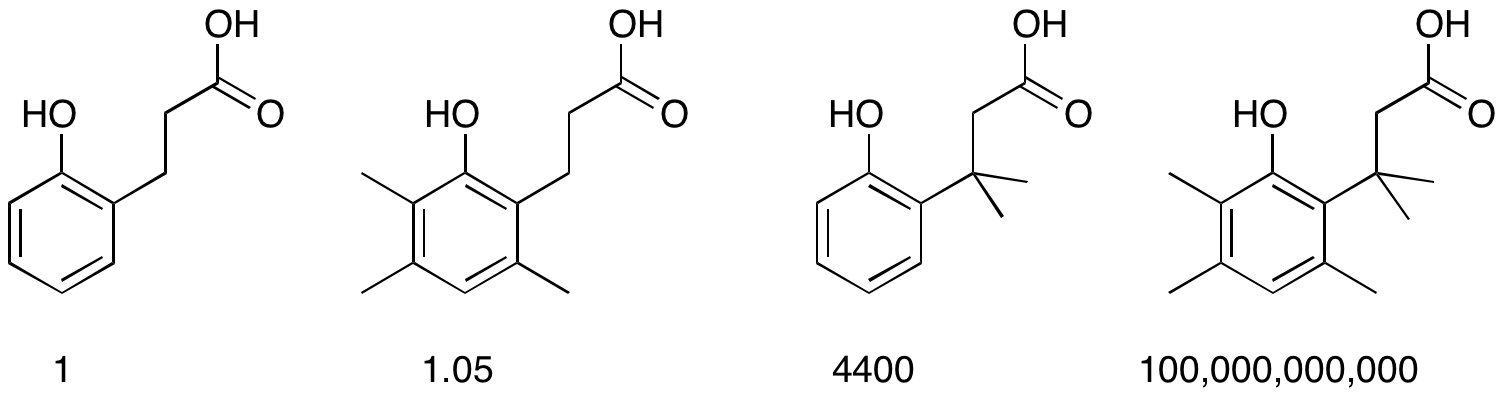
Thorpe–Ingold Effect
The Thorpe–Ingold effect, gem-dimethyl effect, or angle compression is an effect observed in chemistry where increasing steric hindrance favours ring closure and intramolecular reactions. The effect was first reported by Beesley, Thorpe, and Ingold in 1915 as part of a study of cyclization reactions. It has since been generalized to many areas of chemistry. The comparative rates of lactone formation (lactonization) of various 2-hydroxybenzenepropionic acids illustrate the effect. The placement of an increasing number of methyl groups accelerates the cyclization process. : One application of this effect is addition of a quaternary carbon (e.g., a gem-dimethyl group) in an alkyl chain to increase the reaction rate and/or equilibrium constant of cyclization reactions. An example of this is an olefin metathesis reaction: In the field of peptide foldamers, amino acid residues containing quaternary carbons such as 2-aminoisobutyric acid are used to promote formation of certain ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. A polypeptide is a longer, continuous, unbranched peptide chain. Hence, peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. A polypeptide that contains more than approximately 50 amino acids is known as a protein. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, or to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteinogenic Amino Acid
Proteinogenic amino acids are amino acids that are incorporated biosynthetically into proteins during translation. The word "proteinogenic" means "protein creating". Throughout known life, there are 22 genetically encoded (proteinogenic) amino acids, 20 in the standard genetic code and an additional 2 (selenocysteine and pyrrolysine) that can be incorporated by special translation mechanisms. In contrast, non-proteinogenic amino acids are amino acids that are either not incorporated into proteins (like GABA, L-DOPA, or triiodothyronine), misincorporated in place of a genetically encoded amino acid, or not produced directly and in isolation by standard cellular machinery (like hydroxyproline). The latter often results from post-translational modification of proteins. Some non-proteinogenic amino acids are incorporated into nonribosomal peptides which are synthesized by non-ribosomal peptide synthetases. Both eukaryotes and prokaryotes can incorporate selenocysteine into their p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |