|
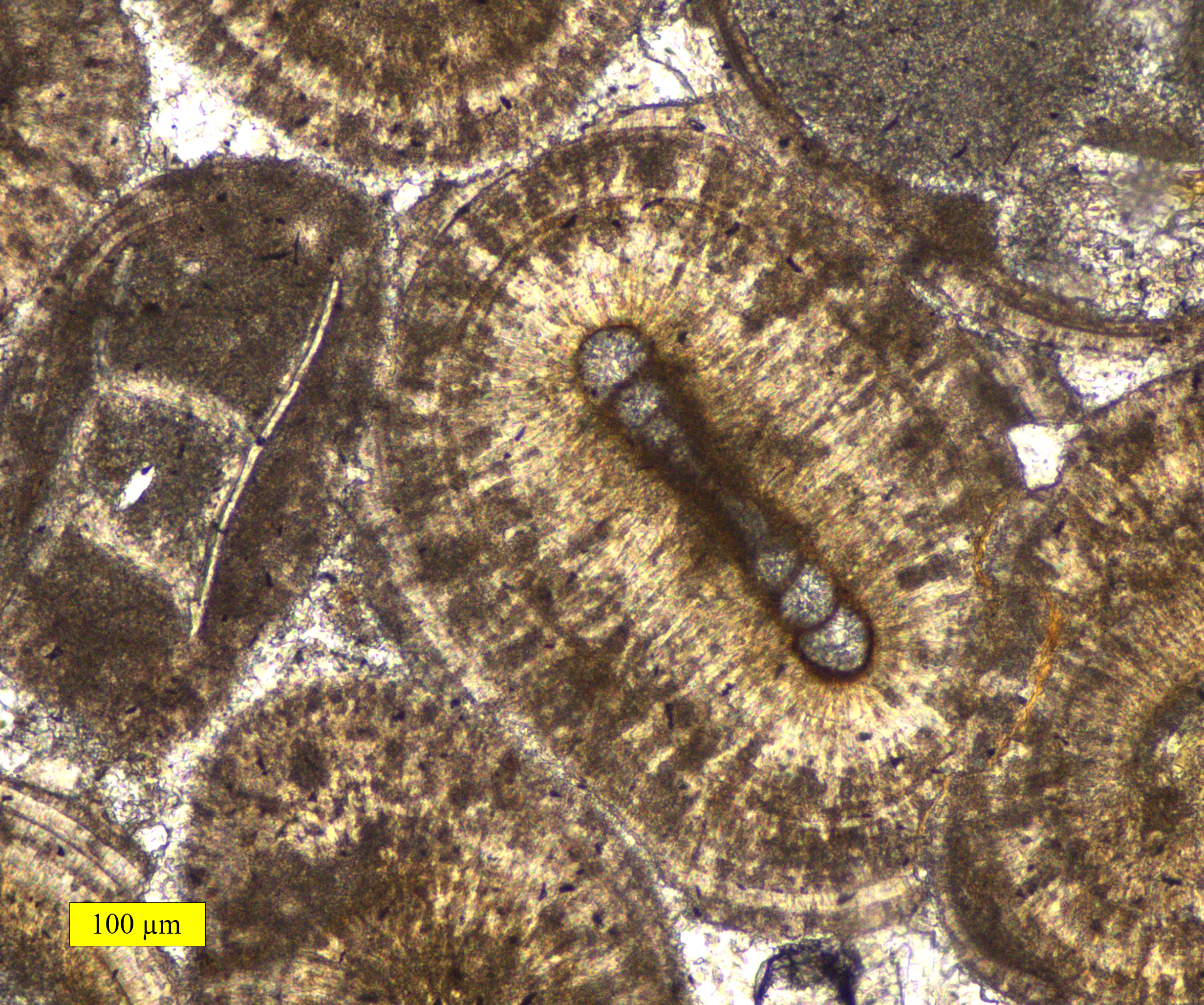
Ooid
Ooids are small (commonly ≤2 mm in diameter), spheroidal, "coated" (layered) sedimentary grains, usually composed of calcium carbonate, but sometimes made up of iron- or phosphate-based minerals. Ooids usually form on the sea floor, most commonly in shallow tropical seas (around the Bahamas, for example, or in the Persian Gulf). After being buried under additional sediment, these ooid grains can be cemented together to form a sedimentary rock called an ''oolite''. Oolites usually consist of calcium carbonate; these belong to the limestone rock family. Pisoids are similar to ooids, but are larger than 2 mm in diameter, often considerably larger, as with the pisoids in the hot springs at Carlsbad (Karlovy Vary) in the Czech Republic. Formation An ooid forms as a series of concentric layers around a nucleus. The layers contain crystals arranged radially, tangentially or randomly. The nucleus can be a shell fragment, quartz grain or any other small fragment. Most mode ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Limestone
Limestone ( calcium carbonate ) is a type of carbonate sedimentary rock which is the main source of the material lime. It is composed mostly of the minerals calcite and aragonite, which are different crystal forms of . Limestone forms when these minerals precipitate out of water containing dissolved calcium. This can take place through both biological and nonbiological processes, though biological processes, such as the accumulation of corals and shells in the sea, have likely been more important for the last 540 million years. Limestone often contains fossils which provide scientists with information on ancient environments and on the evolution of life. About 20% to 25% of sedimentary rock is carbonate rock, and most of this is limestone. The remaining carbonate rock is mostly dolomite, a closely related rock, which contains a high percentage of the mineral dolomite, . ''Magnesian limestone'' is an obsolete and poorly-defined term used variously for dolomite, for limes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oolite
Oolite or oölite (''egg stone'') is a sedimentary rock formed from ooids, spherical grains composed of concentric layers. The name derives from the Ancient Greek word for egg (ᾠόν). Strictly, oolites consist of ooids of diameter 0.25–2 millimetres; rocks composed of ooids larger than 2 mm are called pisolites. The term ''oolith'' can refer to oolite or individual ooids. Composition Ooids are most commonly composed of calcium carbonate ( calcite or aragonite), but can be composed of phosphate, clays, chert, dolomite or iron minerals, including hematite. Dolomitic and chert ooids are most likely the result of the replacement of the original texture in limestone. Oolitic hematite occurs at Red Mountain near Birmingham, Alabama, along with oolitic limestone. They are usually formed in warm, supersaturated, shallow, highly agitated marine water intertidal environments, though some are formed in inland lakes. The mechanism of formation starts with a small fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oolitic Aragonite Sand
Oolitic aragonite sand is composed of the calcium carbonate mineral, aragonite, with an egg-like shape (" oolitic" from the Ancient Greek word ᾠόν for "egg") and sand grain size. This sand type forms in tropical waters through precipitation, sedimentation, and microbial activity, and is indicative of high energy environments. The production of oolitic aragonite sand in the Bahamas surpasses anyplace else in the world. Changes in seawater chemistry and paleoenvironments can be interpreted by the sand's chemical composition and structure. Formation Oolitic aragonite sand forms in high-salinity waters that are turbulent, shallow, and warm. The sand starts to form around a nucleus of calcium carbonate, such as a peloid, shell fragment, or foraminifer. The nucleus is coated with a thin layer of crystalline carbonate to form the cortex of the ooid. Compared to other types of sand formation that involve the weathering and erosion of a larger rock by turbulent waters, oolitic arag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcite
Calcite is a carbonate mineral and the most stable polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on the Mohs scale of mineral hardness, based on scratch hardness comparison. Large calcite crystals are used in optical equipment, and limestone composed mostly of calcite has numerous uses. Other polymorphs of calcium carbonate are the minerals aragonite and vaterite. Aragonite will change to calcite over timescales of days or less at temperatures exceeding 300 °C, and vaterite is even less stable. Etymology Calcite is derived from the German ''Calcit'', a term from the 19th century that came from the Latin word for lime, ''calx'' (genitive calcis) with the suffix "-ite" used to name minerals. It is thus etymologically related to chalk. When applied by archaeologists and stone trade professionals, the term alabaster is used not just as in geology and mineralogy, where it is res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcite Seas
A calcite sea is a sea in which low-magnesium calcite is the primary inorganic marine calcium carbonate precipitate. An aragonite sea is the alternate seawater chemistry in which aragonite and high-magnesium calcite are the primary inorganic carbonate precipitates. The Early Paleozoic and the Middle to Late Mesozoic oceans were predominantly calcite seas, whereas the Middle Paleozoic through the Early Mesozoic and the Cenozoic (including today) are characterized by aragonite seas. The most significant geological and biological effects of calcite sea conditions include rapid and widespread formation of carbonate hardgrounds, calcitic ooids, calcite cements, and the contemporaneous dissolution of aragonite shells in shallow warm seas. Hardgrounds were very common, for example, in the calcite seas of the Ordovician and Jurassic, but virtually absent from the aragonite seas of the Permian. Fossils of invertebrate organisms found in calcite sea deposits are usually dominated by e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jurassic
The Jurassic ( ) is a geologic period and stratigraphic system that spanned from the end of the Triassic Period million years ago (Mya) to the beginning of the Cretaceous Period, approximately Mya. The Jurassic constitutes the middle period of the Mesozoic Era and is named after the Jura Mountains, where limestone strata from the period were first identified. The start of the Jurassic was marked by the major Triassic–Jurassic extinction event, associated with the eruption of the Central Atlantic Magmatic Province. The beginning of the Toarcian Stage started around 183 million years ago and is marked by an extinction event associated with widespread oceanic anoxia, ocean acidification, and elevated temperatures likely caused by the eruption of the Karoo-Ferrar large igneous provinces. The end of the Jurassic, however, has no clear boundary with the Cretaceous and is the only boundary between geological periods to remain formally undefined. By the beginning of the Jurassi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pisolite
A pisolite is a sedimentary rock made of pisoids, which are concretionary grains – typically of calcium carbonate which resemble ooids, but are more than 2 mm in diameter. These grains are approximately spherical and have concentric layers reaching 10 mm in diameter. The name derives from the Hellenic word for pea. Bauxites, limonites, and siderites often have a pisolitic structure. See also * Ooid Ooids are small (commonly ≤2 mm in diameter), spheroidal, "coated" (layered) sedimentary grains, usually composed of calcium carbonate, but sometimes made up of iron- or phosphate-based minerals. Ooids usually form on the sea floor, mo ... * Oolite References Further reading * Sedimentary rocks {{petrology-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcite Sea
A calcite sea is a sea in which low-magnesium calcite is the primary inorganic marine calcium carbonate precipitate. An aragonite sea is the alternate seawater chemistry in which aragonite and high-magnesium calcite are the primary inorganic carbonate precipitates. The Early Paleozoic and the Middle to Late Mesozoic oceans were predominantly calcite seas, whereas the Middle Paleozoic through the Early Mesozoic and the Cenozoic (including today) are characterized by aragonite seas. The most significant geological and biological effects of calcite sea conditions include rapid and widespread formation of carbonate hardgrounds, calcitic ooids, calcite cements, and the contemporaneous dissolution of aragonite shells in shallow warm seas. Hardgrounds were very common, for example, in the calcite seas of the Ordovician and Jurassic, but virtually absent from the aragonite seas of the Permian. Fossils of invertebrate organisms found in calcite sea deposits are usually dominated by e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strontium
Strontium is the chemical element with the symbol Sr and atomic number 38. An alkaline earth metal, strontium is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is exposed to air. Strontium has physical and chemical properties similar to those of its two vertical neighbors in the periodic table, calcium and barium. It occurs naturally mainly in the minerals celestine and strontianite, and is mostly mined from these. Both strontium and strontianite are named after Strontian, a village in Scotland near which the mineral was discovered in 1790 by Adair Crawford and William Cruickshank; it was identified as a new element the next year from its crimson-red flame test color. Strontium was first isolated as a metal in 1808 by Humphry Davy using the then newly discovered process of electrolysis. During the 19th century, strontium was mostly used in the production of sugar from sugar beets (see strontian ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossilised remnants of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name derives from Latin ''calx'' "lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and ph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymorphism (materials Science)
In materials science, polymorphism describes the existence of a solid material in more than one form or crystal structure. Polymorphism is a form of isomerism. Any crystalline material can exhibit the phenomenon. Allotropy refers to polymorphism for chemical elements. Polymorphism is of practical relevance to pharmaceuticals, agrochemicals, pigments, dyestuffs, foods, and explosives. According to IUPAC, a polymorphic transition is "A reversible transition of a solid crystalline phase at a certain temperature and pressure (the inversion point) to another phase of the same chemical composition with a different crystal structure." According to McCrone, polymorphs are "different in crystal structure but identical in the liquid or vapor states." Materials with two polymorphs are called dimorphic, with three polymorphs, trimorphic, etc. Examples Many compounds exhibit polymorphism. It has been claimed that "every compound has different polymorphic forms, and that, in general, th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |