|
Soap Bubble
A soap bubble is an extremely thin film of soap or detergent and water enclosing air that forms a hollow sphere with an iridescent surface. Soap bubbles usually last for only a few seconds before bursting, either on their own or on contact with another object. They are often used for children's enjoyment, but they are also used in artistic performances. Assembling many bubbles results in foam. When light shines onto a bubble it appears to change colour. Unlike those seen in a rainbow, which arise from differential refraction, the colours seen in a soap bubble arise from light wave interference, reflecting off the front and back surfaces of the thin soap film. Depending on the thickness of the film, different colours interfere constructively and destructively. Mathematics Soap bubbles are physical examples of the complex mathematical problem of minimal surface. They will assume the shape of least surface area possible containing a given volume. A true minimal surface is m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reflection In A Soap Bubble Edit
Reflection or reflexion may refer to: Science and technology * Reflection (physics), a common wave phenomenon ** Specular reflection, reflection from a smooth surface *** Mirror image, a reflection in a mirror or in water ** Signal reflection, in signal transmission * Elastic scattering, a process in nuclear and particle physics * Reflection nebula, a nebula that is extended and has no boundaries * Reflection seismology or seismic reflection, a method of exploration geophysics Mathematics * Reflection principle, in set theory * Point reflection, a reflection across a point * Reflection (mathematics), a transformation of a space * Reflection formula, a relation in a function * Reflective subcategory, in category theory Computing * Reflection (computer graphics), simulation of reflective surfaces * Reflection (computer programming), a program that accesses or modifies its own code * Reflection, terminal emulation software by Attachmate Arts and entertainment Film and television * T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Bubble Conjecture
In the mathematical theory of minimal surfaces, the double bubble theorem states that the shape that encloses and separates two given volumes and has the minimum possible surface area is a ''standard double bubble'': three spherical surfaces meeting at angles of 120° on a common circle. The double bubble theorem was formulated and thought to be true in the 19th century, and became a "serious focus of research" by 1989, but was not proven until 2002. The proof combines multiple ingredients. Compactness of rectifiable currents (a generalized definition of surfaces) shows that a solution exists. A symmetry argument proves that the solution must be a surface of revolution, and it can be further restricted to having a bounded number of smooth pieces. Jean Taylor proof of Plateau's laws describes how these pieces must be shaped and connected to each other, and a final case analysis shows that, among surfaces of revolution connected in this way, only the standard double bubble has lo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Washing-up Liquid
Dishwashing liquid (or washing-up liquid in British English), also known as dishwashing soap, dish detergent, and dish soap is a detergent used to assist in dishwashing. It is usually a highly-foaming mixture of surfactants with low skin irritation, and is primarily used for hand washing of glasses, plates, cutlery, and cooking utensils in a sink or bowl. In addition to its primary use, dishwashing liquid also has various informal applications, such as for creating bubbles, clothes washing and cleaning oil-affected birds. History Washing soda (sodium carbonate) is used for dishwashing, and may be used in areas with hard water. It was used for dishwashing before detergents were invented in Germany during World War I. Liquid detergent used for dishwashing was first manufactured in the middle of the 20th century. Dishwashing detergent producers started production in the United States in the 1930–1940s. Teepol, the first such in Europe, commenced production in 1942. In 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycerol
Glycerol (), also called glycerine in British English and glycerin in American English, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known as glycerides. Because it has antimicrobial and antiviral properties, it is widely used in wound and burn treatments approved by the U.S. Food and Drug Administration. Conversely, it is also used as a bacterial culture medium. It can be used as an effective marker to measure liver disease. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature. Structure Although achiral, glycerol is prochiral with respect to reactions of one of the two primary alcohols. Thus, in substituted derivatives, the stereospecific numbering labels the molecule with a "sn-" prefix before the stem name of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Evaporation
Evaporation is a type of vaporization that occurs on the surface of a liquid as it changes into the gas phase. High concentration of the evaporating substance in the surrounding gas significantly slows down evaporation, such as when humidity affects rate of evaporation of water. When the molecules of the liquid collide, they transfer energy to each other based on how they collide. When a molecule near the surface absorbs enough energy to overcome the vapor pressure, it will escape and enter the surrounding air as a gas. When evaporation occurs, the energy removed from the vaporized liquid will reduce the temperature of the liquid, resulting in evaporative cooling. On average, only a fraction of the molecules in a liquid have enough heat energy to escape from the liquid. The evaporation will continue until an equilibrium is reached when the evaporation of the liquid is equal to its condensation. In an enclosed environment, a liquid will evaporate until the surrounding ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Capillary Length
The capillary length or capillary constant, is a length scaling factor that relates gravity and surface tension. It is a fundamental physical property that governs the behavior of menisci, and is found when body forces (gravity) and surface forces ( Laplace pressure) are in equilibrium. The pressure of a static fluid does not depend on the shape, total mass or surface area of the fluid. It is directly proportional to the fluid's specific weight – the force exerted by gravity over a specific volume, and its vertical height. However, a fluid also experiences pressure that is induced by surface tension, commonly referred to as the Young-Laplace pressure. Surface tension originates from cohesive forces between molecules, and in the bulk of the fluid, molecules experience attractive forces from all directions. The surface of a fluid is curved because exposed molecules on the surface have fewer neighboring interactions, resulting in a net force that contracts the surface. There exists ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
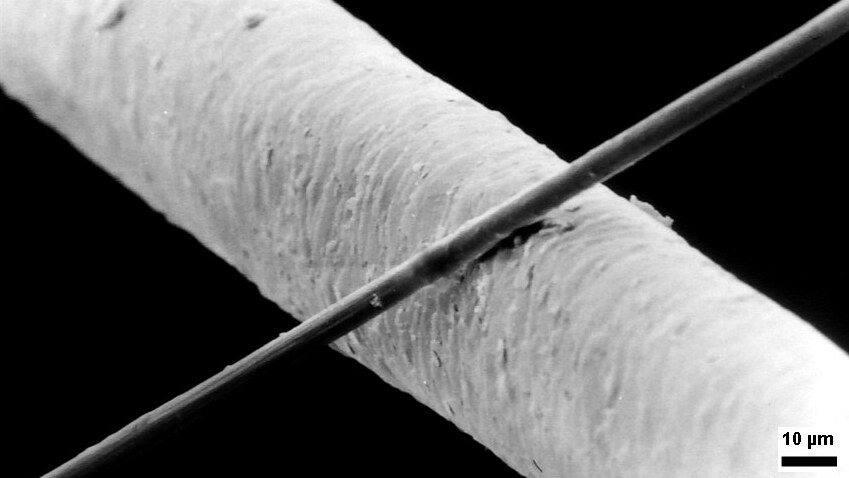
Micrometre
The micrometre (American and British English spelling differences#-re, -er, international spelling as used by the International Bureau of Weights and Measures; SI symbol: μm) or micrometer (American and British English spelling differences#-re, -er, American spelling), also commonly known as a micron, is a unit of length in the International System of Units (SI) equalling (SI standard prefix "micro-" = ); that is, one millionth of a metre (or one thousandth of a millimetre, , or about ). The nearest smaller common SI unit is the nanometre, equivalent to one thousandth of a micrometre, one millionth of a millimetre or one billionth of a metre (). The micrometre is a common unit of measurement for wavelengths of infrared radiation as well as sizes of biological cell (biology), cells and bacteria, and for grading wool by the diameter of the fibres. The width of a single human hair ranges from approximately 20 to . The longest human chromosome, chromosome 1 (human), chromosome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plateau's Laws
Plateau's laws describe the structure of soap films. These laws were formulated in the 19th century by the Belgian physicist Joseph Plateau from his experimental observations. Many patterns in nature are based on foams obeying these laws. Laws for soap films Plateau's laws describe the shape and configuration of soap films as follows: # Soap films are made of entire (unbroken) smooth surfaces. # The mean curvature of a portion of a soap film is everywhere constant on any point on the same piece of soap film. # Soap films always meet in threes along an edge called a Plateau border, and they do so at an angle of arccos(−) = 120°. # These Plateau borders meet in fours at a vertex, at the tetrahedral angle In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all th ... of arccos(−)&nb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Young–Laplace Equation
In physics, the Young–Laplace equation () is an algebraic equation that describes the capillary pressure difference sustained across the interface between two static fluids, such as water and air, due to the phenomenon of surface tension or wall tension, although use of the latter is only applicable if assuming that the wall is very thin. The Young–Laplace equation relates the pressure difference to the shape of the surface or wall and it is fundamentally important in the study of static capillary surfaces. It's a statement of normal stress balance for static fluids meeting at an interface, where the interface is treated as a surface (zero thickness): \begin \Delta p &= -\gamma \nabla \cdot \hat n \\ &= -2\gamma H_f \\ &= -\gamma \left(\frac + \frac\right) \end where \Delta p is the Laplace pressure, the pressure difference across the fluid interface (the exterior pressure minus the interior pressure), \gamma is the surface tension (or wall tension), \hat n is the unit no ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and even by industry. Further, both spellings are often used ''within'' a particular industry or country. Industries in British English-speaking countries typically use the "gauge" spelling. is the pressure relative to the ambient pressure. Various units are used to express pressure. Some of these derive from a unit of force divided by a unit of area; the SI unit of pressure, the pascal (Pa), for example, is one newton per square metre (N/m2); similarly, the pound-force per square inch ( psi) is the traditional unit of pressure in the imperial and U.S. customary systems. Pressure may also be expressed in terms of standard atmospheric pressure; the atmosphere (atm) is equal to this pressure, and the torr is defined as of this. Man ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Soap Bubbles Being Formed By A Bubble Wand - Slow Motion - 2022 July 28
Soap is a salt of a fatty acid used in a variety of cleansing and lubricating products. In a domestic setting, soaps are surfactants usually used for washing, bathing, and other types of housekeeping. In industrial settings, soaps are used as thickeners, components of some lubricants, and precursors to catalysts. When used for cleaning, soap solubilizes particles and grime, which can then be separated from the article being cleaned. In hand washing, as a surfactant, when lathered with a little water, soap kills microorganisms by disorganizing their membrane lipid bilayer and denaturing their proteins. It also emulsifies oils, enabling them to be carried away by running water. Soap is created by mixing fats and oils with a base. A similar process is used for making detergent which is also created by combining chemical compounds in a mixer. Humans have used soap for millennia. Evidence exists for the production of soap-like materials in ancient Babylon around 2800 BC. Ty ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ggb In Soap Bubble 1
GGB may refer to: * Gerdau, a Brazilian steel company * GGB Bearing Technology, a global plain bearings manufacturer * Golden Gate Bridge, a suspension bridge in San Francisco, CA * Gornergrat railway (German: '), in Switzerland * Green Garter Band The Green Garter Band (GGB) is a group of twelve that plays at numerous events for the University of Oregon. The group has a faculty advisor, the Director of Athletic Bands, but for the most part is run by its student members. The band performs f ..., of the University of Oregon * Grupo Gay da Bahia, a gay rights organization in Brazil {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |





.jpg)
